Therapeutic effect of Echinococcus granulosus cyst fluid on bacterial sepsis in mice
- PMID: 38066526
- PMCID: PMC10709918
- DOI: 10.1186/s13071-023-06021-7
Therapeutic effect of Echinococcus granulosus cyst fluid on bacterial sepsis in mice
Abstract
Background: The primary pathophysiological process of sepsis is to stimulate a massive release of inflammatory mediators to trigger systemic inflammatory response syndrome (SIRS), the major cause of multi-organ dysfunction and death. Like other helminths, Echinococcus granulosus induces host immunomodulation. We sought to determine whether E. granulosus cyst fluid (EgCF) displays a therapeutic effect on sepsis-induced inflammation and tissue damage in a mouse model.
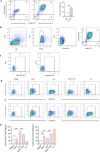
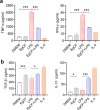
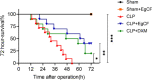
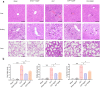
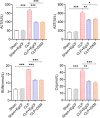
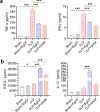
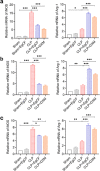
Methods: The anti-inflammatory effects of EgCF were determined by in vitro culture with bone marrow-derived macrophages (BMDMs) and in vivo treatment of BALB/C mice with cecal ligation and puncture (CLP)-induced sepsis. The macrophage phenotypes were determined by flow cytometry, and the levels of cytokines in cell supernatants or in sera of mice were measured (ELISA). The therapeutic effect of EgCF on sepsis was evaluated by observing the survival rates of mice for 72 h after CLP, and the pathological injury to the liver, kidney, and lung was measured under a microscope. The expression of TLR-2/MyD88 in tissues was measured by western blot to determine whether TLR-2/MyD88 is involved in the sepsis-induced inflammatory signaling pathway.
Results: In vitro culture with BMDMs showed that EgCF promoted macrophage polarization to M2 type and inhibited lipopolysaccharide (LPS)-induced M1 macrophages. EgCF treatment provided significant therapeutic effects on CLP-induced sepsis in mice, with increased survival rates and alleviation of tissue injury. The EgCF conferred therapeutic efficacy was associated with upregulated anti-inflammatory cytokines (IL-10 and TGF-β) and reduced pro-inflammatory cytokines (TNF-α and INF-γ). Treatment with EgCF induced Arg-1-expressed M2, and inhibited iNOS-expressed M1 macrophages. The expression of TLR-2 and MyD88 in EgCF-treated mice was reduced.
Conclusions: The results demonstrated that EgCF confers a therapeutic effect on sepsis by inhibiting the production of pro-inflammatory cytokines and inducing regulatory cytokines. The anti-inflammatory effect of EgCF is carried out possibly through inducing macrophage polarization from pro-inflammatory M1 to regulatory M2 phenotype to reduce excessive inflammation of sepsis and subsequent multi-organ damage. The role of EgCF in regulating macrophage polarization may be achieved by inhibiting the TLR2/MyD88 signaling pathway.
Keywords: Cyst fluid; Echinococcus granulosus; Immunomodulation; Macrophage; Sepsis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Therapeutic effect of recombinant Echinococcus granulosus antigen B subunit 2 protein on sepsis in a mouse model.Parasit Vectors. 2024 Nov 15;17(1):467. doi: 10.1186/s13071-024-06540-x. Parasit Vectors. 2024. PMID: 39548530 Free PMC article.
-
Trichinella spiralis cystatin alleviates polymicrobial sepsis through activating regulatory macrophages.Int Immunopharmacol. 2022 Aug;109:108907. doi: 10.1016/j.intimp.2022.108907. Epub 2022 Jun 9. Int Immunopharmacol. 2022. PMID: 35691271
-
Therapeutic effect of Schistosoma japonicum cystatin on bacterial sepsis in mice.Parasit Vectors. 2017 May 8;10(1):222. doi: 10.1186/s13071-017-2162-0. Parasit Vectors. 2017. PMID: 28482922 Free PMC article.
-
Growth Differentiation Factor-15 Orchestrates Inflammation-Related Diseases via Macrophage Polarization.Discov Med. 2024 Feb;36(181):248-255. doi: 10.24976/Discov.Med.202436181.23. Discov Med. 2024. PMID: 38409830 Review.
-
[Role of macrophage polarization in hypertension and traditional Chinese medicine intervention: a review].Zhongguo Zhong Yao Za Zhi. 2024 Jan;49(1):15-25. doi: 10.19540/j.cnki.cjcmm.20231016.601. Zhongguo Zhong Yao Za Zhi. 2024. PMID: 38403334 Review. Chinese.
Cited by
-
Therapeutic effect of recombinant Echinococcus granulosus antigen B subunit 2 protein on sepsis in a mouse model.Parasit Vectors. 2024 Nov 15;17(1):467. doi: 10.1186/s13071-024-06540-x. Parasit Vectors. 2024. PMID: 39548530 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- Z202114/the Major Projects of Wuxi Municipal Health and Wellness Commission
- by51201306/512 Talents Development Project of Bengbu Medical College
- by51201205/512 Talents Development Project of Bengbu Medical College
- gxyq2021188/the Support Program for Excellent Talents in Higher Educational Institutions in Anhui Province
- AHWJ2021a005/the Key Projects of Scientific Research of Anhui Provincial Health and Wellness Commission
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

