A PTHrP Gradient Drives Mandibular Condylar Chondrogenesis via Runx2
- PMID: 38058151
- PMCID: PMC10734211
- DOI: 10.1177/00220345231208175
A PTHrP Gradient Drives Mandibular Condylar Chondrogenesis via Runx2
Abstract
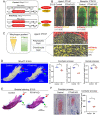
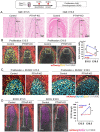
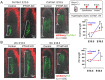
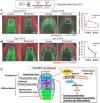
The mandibular condylar cartilage (MCC) is an essential component of the temporomandibular joint, which orchestrates the vertical growth of the mandibular ramus through endochondral ossification with distinctive modes of cell differentiation. Parathyroid hormone-related protein (PTHrP) is a master regulator of chondrogenesis; in the long bone epiphyseal growth plate, PTHrP expressed by resting zone chondrocytes promotes chondrocyte proliferation in the adjacent layer. However, how PTHrP regulates chondrogenesis in the MCC remains largely unclear. In this study, we used a Pthrp-mCherry knock-in reporter strain to map the localization of PTHrP+ cells in the MCC and define the function of PTHrP in the growing mandibular condyle. In the postnatal MCC of PthrpmCherry/+ mice, PTHrP-mCherry was specifically expressed by cells in the superficial layer immediately adjacent to RUNX2-expressing cells in the polymorphic layer. PTHrP ligands diffused across the polymorphic and chondrocyte layers where its cognate receptor PTH1R was abundantly expressed. We further analyzed the mandibular condyle of PthrpmCherry/mCherry mice lacking functional PTHrP protein (PTHrP-KO). At embryonic day (E) 18.5, the condylar process and MCC were significantly truncated in the PTHrP-KO mandible, which was associated with a significant reduction in cell proliferation across the polymorphic layer and a loss of SOX9+ cells in the chondrocyte layers. The PTHrP-KO MCC showed a transient increase in the number of Col10a1+ hypertrophic chondrocytes at E15.5, followed by a significant loss of these cells at E18.5, indicating that superficial layer-derived PTHrP prevents premature chondrocyte exhaustion in the MCC. The expression of Runx2, but not Sp7, was significantly reduced in the polymorphic layer of the PTHrP-KO MCC. Therefore, PTHrP released from cells in the superficial layer directly acts on cells in the polymorphic layer to promote proliferation of chondrocyte precursor cells and prevent their premature differentiation by maintaining Runx2 expression, revealing a unique PTHrP gradient-directed mechanism that regulates MCC chondrogenesis.
Keywords: articular cartilage; bone development; chondrocytes; mandibular condylar cartilage (MCC); periosteum; temporomandibular joint.
Conflict of interest statement
Declaration of Conflicting InterestsThe authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

Similar articles
-
Parathyroid hormone-related protein regulates proliferation of condylar hypertrophic chondrocytes.J Bone Miner Res. 1999 Nov;14(11):1838-47. doi: 10.1359/jbmr.1999.14.11.1838. J Bone Miner Res. 1999. PMID: 10571683
-
Deletion of Runx2 in condylar chondrocytes disrupts TMJ tissue homeostasis.J Cell Physiol. 2019 Apr;234(4):3436-3444. doi: 10.1002/jcp.26761. Epub 2018 Nov 1. J Cell Physiol. 2019. PMID: 30387127 Free PMC article.
-
Local injection of abaloparatide promotes mandibular condyle lengthening in adolescent rats via enhancing chondrogenesis and ossification.J Oral Rehabil. 2024 Feb;51(2):380-393. doi: 10.1111/joor.13597. Epub 2023 Sep 19. J Oral Rehabil. 2024. PMID: 37727017
-
The Roles of Indian Hedgehog Signaling in TMJ Formation.Int J Mol Sci. 2019 Dec 13;20(24):6300. doi: 10.3390/ijms20246300. Int J Mol Sci. 2019. PMID: 31847127 Free PMC article. Review.
-
Inhibitory function of parathyroid hormone-related protein on chondrocyte hypertrophy: the implication for articular cartilage repair.Arthritis Res Ther. 2012 Aug 31;14(4):221. doi: 10.1186/ar4025. Arthritis Res Ther. 2012. PMID: 22971952 Free PMC article. Review.
References
-
- Bender ME, Lipin RB, Goudy SL. 2018. Development of the pediatric temporomandibular joint. Oral Maxillofac Surg Clin North Am. 30(1):1–9. - PubMed
-
- Gurdon JB, Bourillot PY. 2001. Morphogen gradient interpretation. Nature. 413(6858):797–803. - PubMed
-
- Hinton RJ. 2014. Genes that regulate morphogenesis and growth of the temporomandibular joint: a review. Dev Dyn. 243(7):864–874. - PubMed
-
- Kantomaa T, Tuominen M, Pirttiniemi P. 1994. Effect of mechanical forces on chondrocyte maturation and differentiation in the mandibular condyle of the rat. J Dent Res. 73(6):1150–1156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

