Ontogenesis of the Mouse Ocular Surface Lymphatic Vascular Network
- PMID: 38054922
- PMCID: PMC10702784
- DOI: 10.1167/iovs.64.15.7
Ontogenesis of the Mouse Ocular Surface Lymphatic Vascular Network
Abstract
Purpose: Ocular lymphatic vessels play major physiological role in eye homeostasis and their dysfunction can contribute to the progression of several eye diseases. In this study, we characterized their spatiotemporal development and the cellular mechanisms occurring during their ontogenesis in the mouse eye.
Methods: Whole mount immunofluorescent staining and imaging by standard or lightsheet fluorescence microscopy were performed on late embryonic and early postnatal eye mouse samples.
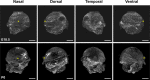
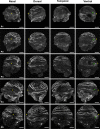
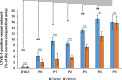
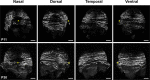
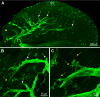
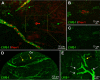
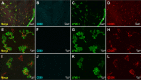
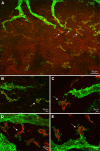
Results: We observed that the ocular surface lymphatic vascular network develops at the early postnatal stages (between P0 and P5) from two nascent trunks arising at the nasal side on both sides of the nictitating membrane. These nascent vessels further branch and encircle the whole eye surface by sprouting lymphangiogenesis. In addition, we got evidence for the existence of a transient lymphvasculogenesis process generating lymphatic vessel fragments that will mostly formed the corneolimbal lymphatic vasculature which further connect to the conjunctival lymphatic network. Our results also support that CD206-positive macrophages can transdifferentiate and then integrate into the lymphatic neovessels.
Conclusions: Several complementary cellular processes participate in the development of the lymphatic ocular surface vasculature. This knowledge paves the way for the design of new therapeutic strategies to interfere with ocular lymphatic vessel formation when needed.
Conflict of interest statement
Disclosure:
Figures
Similar articles
-
LYVE-1-positive macrophages are present in normal murine eyes.Invest Ophthalmol Vis Sci. 2007 May;48(5):2162-71. doi: 10.1167/iovs.06-0783. Invest Ophthalmol Vis Sci. 2007. PMID: 17460275
-
The Impact of Stem/Progenitor Cells on Lymphangiogenesis in Vascular Disease.Cells. 2022 Dec 15;11(24):4056. doi: 10.3390/cells11244056. Cells. 2022. PMID: 36552820 Free PMC article. Review.
-
Histological and Morphological Characterization of Developing Dermal Lymphatic Vessels.Methods Mol Biol. 2018;1846:19-35. doi: 10.1007/978-1-4939-8712-2_2. Methods Mol Biol. 2018. PMID: 30242750
-
Regulation of lymphangiogenesis in the diaphragm by macrophages and VEGFR-3 signaling.Angiogenesis. 2016 Oct;19(4):513-24. doi: 10.1007/s10456-016-9523-8. Epub 2016 Jul 27. Angiogenesis. 2016. PMID: 27464987 Free PMC article.
-
Lymphatic vessels of the eye - old questions - new insights.Ann Anat. 2019 Jan;221:1-16. doi: 10.1016/j.aanat.2018.08.004. Epub 2018 Sep 18. Ann Anat. 2019. PMID: 30240907 Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

