Inhaled corticosteroids with combination inhaled long-acting beta2-agonists and long-acting muscarinic antagonists for chronic obstructive pulmonary disease
- PMID: 38054551
- PMCID: PMC10698842
- DOI: 10.1002/14651858.CD011600.pub3
Inhaled corticosteroids with combination inhaled long-acting beta2-agonists and long-acting muscarinic antagonists for chronic obstructive pulmonary disease
Abstract
Background: Management of chronic obstructive pulmonary disease (COPD) commonly involves a combination of long-acting bronchodilators including beta2-agonists (LABA) and muscarinic antagonists (LAMA). LABA and LAMA bronchodilators are now available in single-combination inhalers. In individuals with persistent symptoms or frequent exacerbations, inhaled corticosteroids (ICS) are also used with combination LABA and LAMA inhalers. However, the benefits and risks of adding ICS to combination LABA/LAMA inhalers as a triple therapy remain unclear.
Objectives: To assess the effects of adding an ICS to combination LABA/LAMA inhalers for the treatment of stable COPD.
Search methods: We searched the Cochrane Airways Group Register of Trials, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and Embase up to 30 November 2022. We also searched ClinicalTrials.gov and the WHO ICTRP up to 30 November 2022.
Selection criteria: We included parallel-group randomised controlled trials of three weeks' duration or longer that compared the treatment of stable COPD with ICS in addition to combination LABA/LAMA inhalers against combination LABA/LAMA inhalers alone.
Data collection and analysis: We used standard Cochrane methodological procedures. The primary outcomes were acute exacerbations of COPD, respiratory health-related quality of life, pneumonia and other serious adverse events. The secondary outcomes were symptom scores, lung function, physical capacity, and mortality. We used GRADE to assess certainty of evidence for studies that contributed data to our prespecified outcomes.
Main results: Four studies with a total of 15,412 participants met the inclusion criteria. The mean age of study participants ranged from 64.4 to 65.3 years; the proportion of female participants ranged from 28% to 40%. Most participants had symptomatic COPD (COPD Assessment Test Score ≥ 10) with severe to very severe airflow limitation (forced expiratory volume in one second (FEV1) < 50% predicted) and one or more moderate-to-severe COPD exacerbations in the last 12 months. Trial medications differed amongst studies. The duration of follow-up was 52 weeks in three studies and 24 weeks in one study. We assessed the risk of selection, performance, and detection bias to be low in the included studies; one study was at high risk of attrition bias, and one study was at high risk of reporting bias. Triple therapy may reduce rates of moderate-to-severe COPD exacerbations compared to combination LABA/LAMA inhalers (rate ratio (RR) 0.74, 95% confidence interval (CI) 0.67 to 0.81; n = 15,397; low-certainty evidence). Subgroup analysis stratifying by blood eosinophil counts showed there may be a greater reduction in rate of moderate-to-severe COPD exacerbations with triple therapy in participants with high-eosinophils (RR 0.67, 95% CI 0.60 to 0.75) compared to low-eosinophils (RR 0.87, 95% CI 0.81 to 0.93) (test for subgroup differences: P < 0.01) (high/low cut-offs: 150 eosinophils/µL in three studies; 200 eosinophils/µL in one study). However, moderate-to-substantial heterogeneity was observed in both high- and low-eosinophil subgroups. These subgroup analyses are observational by nature and thus results should be interpreted with caution. Triple therapy may be associated with reduced rates of severe COPD exacerbations (RR 0.75, 95% CI 0.67 to 0.84; n = 14,131; low-certainty evidence). Triple therapy improved health-related quality of life assessed using the St George's Respiratory Questionnaire (SGRQ) by the minimal clinically important difference (MCID) threshold (4-point decrease) (35.3% versus 42.4%, odds ratio (OR) 1.35, 95% CI 1.26 to 1.45; n = 14,070; high-certainty evidence). Triple therapy may result in fewer symptoms measured using the Transition Dyspnoea Index (TDI) (OR 1.33, 95% CI 1.13 to 1.57; n = 3044; moderate-certainty evidence) and improved lung function as measured by change in trough FEV1 (mean difference 38.68 mL, 95% CI 22.58 to 54.77; n = 11,352; low-certainty evidence). However, these benefits fell below MCID thresholds for TDI (1-unit decrease) and trough FEV1 (100 mL), respectively. Triple therapy is probably associated with a higher risk of pneumonia as a serious adverse event compared to combination LABA/LAMA inhalers (3.3% versus 1.9%, OR 1.74, 95% CI 1.39 to 2.18; n = 15,412; moderate-certainty evidence). In contrast, all-cause serious adverse events may be similar between groups (19.7% versus 19.7%, OR 0.95, 95% CI 0.87 to 1.03; n = 15,412; low-certainty evidence). All-cause mortality may be lower with triple therapy (1.4% versus 2.0%, OR 0.70, 95% CI 0.54 to 0.90; n = 15,397; low-certainty evidence).
Authors' conclusions: The available evidence suggests that triple therapy may reduce rates of COPD exacerbations (low-certainty evidence) and results in an improvement in health-related quality of life (high-certainty evidence) compared to combination LABA/LAMA inhalers, but probably confers an increased pneumonia risk as a serious adverse event (moderate-certainty evidence). Triple therapy probably improves respiratory symptoms and may improve lung function (moderate- and low-certainty evidence, respectively); however, these benefits do not appear to be clinically significant. Triple therapy may reduce the risk of all-cause mortality compared to combination LABA/LAMA inhalers (low-certainty evidence). The certainty of the evidence was downgraded most frequently for inconsistency or indirectness. Across the four included studies, there were important differences in inclusion criteria, trial medications, and duration of follow-up. Investigation of heterogeneity was limited due to the small number of included studies. We found limited data on the effects of triple therapy compared to combination LABA/LAMA inhalers in patients with mild-moderate COPD and those without a recent exacerbation history.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Wouter H van Geffen: Fiduciary Officer, Long Range Planning Committee ERS Assembly 11 – Thoracic Oncology, European Respiratory Society (ERS); Board Member, Dutch Society of Respiratory Physicians (NVALT); Consultant Respiratory Physician, Medical Centre Leeuwarden; affiliated to ERS and NVALT; member, Editorial Board of Cochrane Airways
Daniel J Tan: no relevant interests; General Medicine Registrar at the Royal Melbourne Hospital, Melbourne, Victoria, Australia.
Julia AE Walters: none known.
E Haydn Walters: no relevant interests; Acute Physician, Alfred Hospital Melbourne.
Figures
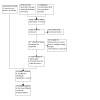

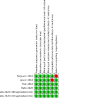













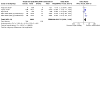
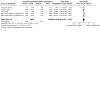



































Update of
-
Inhaled corticosteroids with combination inhaled long-acting beta2-agonists and long-acting muscarinic antagonists for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2016 Nov 10;11(11):CD011600. doi: 10.1002/14651858.CD011600.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2023 Dec 6;12:CD011600. doi: 10.1002/14651858.CD011600.pub3. PMID: 27830584 Free PMC article. Updated. Review.
Similar articles
-
Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2012 Sep 12;2012(9):CD006829. doi: 10.1002/14651858.CD006829.pub2. Cochrane Database Syst Rev. 2012. PMID: 22972099 Free PMC article. Review.
-
Long-acting beta2-agonists for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2013 Oct 15;2013(10):CD010177. doi: 10.1002/14651858.CD010177.pub2. Cochrane Database Syst Rev. 2013. PMID: 24127118 Free PMC article. Review.
-
Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article.
-
Topical and oral steroids for otitis media with effusion (OME) in children.Cochrane Database Syst Rev. 2023 Dec 13;12(12):CD015255. doi: 10.1002/14651858.CD015255.pub2. Cochrane Database Syst Rev. 2023. PMID: 38088821 Free PMC article.
-
Combined corticosteroid and long-acting beta₂-agonist in one inhaler versus placebo for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2013 Nov 10;2013(11):CD003794. doi: 10.1002/14651858.CD003794.pub4. Cochrane Database Syst Rev. 2013. PMID: 24214176 Free PMC article. Review.
Cited by
-
Inhaled corticosteroid treatment and pneumonia in patients with chronic obstructive pulmonary disease - nationwide development from 1998 to 2018.Eur Clin Respir J. 2024 May 29;11(1):2359768. doi: 10.1080/20018525.2024.2359768. eCollection 2024. Eur Clin Respir J. 2024. PMID: 38817947 Free PMC article.
-
Interplay between Lung Diseases and Viral Infections: A Comprehensive Review.Microorganisms. 2024 Oct 8;12(10):2030. doi: 10.3390/microorganisms12102030. Microorganisms. 2024. PMID: 39458339 Free PMC article. Review.
-
Survival benefit of inhaled corticosteroids in patients with chronic obstructive pulmonary disease: a nationwide cohort study.Sci Rep. 2024 Jun 26;14(1):14703. doi: 10.1038/s41598-024-65763-1. Sci Rep. 2024. PMID: 38926519 Free PMC article.
References
References to studies included in this review
Ferguson 2018 {published data only}
-
- Ferguson GT, Rabe KF, Martinez FJ, Fabbri LM, Wang C, Ichinose M, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respiratory Medicine 2018;6(10):747-58. - PubMed
Lipson 2018 {published and unpublished data}
-
- Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. New England Journal of Medicine 2018;378(18):1671-80. - PubMed
-
- Pascoe SJ, Lipson DA, Locantore N, Barnacle H, Brealey N, Mohindra R, et al. A phase III randomised controlled trial of single-dose triple therapy in COPD: the IMPACT protocol. European Respiratory Journal 2016;48(2):320-30. - PubMed
Papi 2018 {published data only}
-
- Papi A, Vestbo J, Fabbri L, Corradi M, Prunier H, Cohuet G, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet 2018;391(10125):1076-84. - PubMed
Rabe 2020 {published data only}
-
- Rabe KF, Martinez FJ, Ferguson GT, Wang C, Singh D, Wedzicha JA, et al. A phase III study of triple therapy with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler 320/18/9.6mug and 160/18/9.6mug using co-suspension delivery technology in moderate-to-very severe COPD: the ETHOS study protocol. Respiratory Medicine 2019;158:59-66. - PubMed
-
- Rabe KF, Martinez FJ, Ferguson GT, Wang C, Singh D, Wedzicha JA, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. New England Journal of Medicine 2020;383:35-48. - PubMed
Rabe 2020 (160 μg budesonide) {published data only}
-
- Rabe KF, Martinez FJ, Ferguson GT, Wang C, Singh D, Wedzicha JA, et al. A phase III study of triple therapy with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler 320/18/9.6mug and 160/18/9.6mug using co-suspension delivery technology in moderate-to-very severe COPD: the ETHOS study protocol. Respiratory Medicine 2019;158:59-66. - PubMed
-
- Rabe KF, Martinez FJ, Ferguson GT, Wang C, Singh D, Wedzicha JA, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. New England Journal of Medicine 2020;383:35-48. - PubMed
Rabe 2020 (320 μg budesonide) {published data only}
-
- Rabe KF, Martinez FJ, Ferguson GT, Wang C, Singh D, Wedzicha JA, et al. A phase III study of triple therapy with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler 320/18/9.6mug and 160/18/9.6mug using co-suspension delivery technology in moderate-to-very severe COPD: the ETHOS study protocol. Respiratory Medicine 2019;158:59-66. - PubMed
-
- Rabe KF, Martinez FJ, Ferguson GT, Wang C, Singh D, Wedzicha JA, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. New England Journal of Medicine 2020;383:35-48. - PubMed
References to studies excluded from this review
Aaron 2007 {published data only}
-
- Aaron SD, Vandemhee KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Annals of Internal Medicine 2007;146(8):545-55. - PubMed
Anonymous 2017 {published data only}
-
- Single inhaler triple therapy for COPD. Drug and Therapeutics Bulletin 2017;55(12):138-141. - PubMed
Antohe 2018 {published data only}
-
- Antohe I, Antoniu SA, Gavrilovici C. Triple fixed inhaled therapy in frequent chronic obstructive pulmonary disease exacerbators: potential advantages for various degrees of airways obstruction. Expert Opinion on Pharmacotherapy 2018;19(3):287-9. - PubMed
Bateman 2015 {published data only}
-
- Bateman ED, Chapman KR, Rennard SI, Lakkis H, Moya M, Garcia Gil E. Efficacy and safety of aclidinium bromide/formoterol fumarate fixed-dose combination in patients with moderate to severe COPD, stratified by inhaled corticosteroid use. American Journal of Respiratory and Critical Care Medicine 2015;191:A5782.
Bishwakarma 2017 {published data only}
Bölükbas 2011 {published data only}
-
- Bölükbas S, Eberlein M, Eckhoff J, Schirren J. Short-term effects of inhalative tiotropium/formoterol/budesonide versus tiotropium/formoterol in patients with newly diagnosed chronic obstructive pulmonary disease requiring surgery for lung cancer: a prospective randomised trial. European Journal of Cardio-Thoracic Surgery 2011;39(6):995-1000. - PubMed
Chapman 2018a {published data only}
-
- Chapman KR, Hurst J, Frent S, Larbig M, Fogel R, Guerin T, et al. Withdrawal of inhaled corticosteroids from COPD patients inhaling long-term triple therapy: the SUNSET study. American Journal of Respiratory and Critical Care Medicine 2018;197:A1009. - PubMed
Chapman 2018b {published data only}
-
- Chapman KR, Hurst JR, Frent S-M, Larbig M, Fogel R, Guerin T, et al. Long-term triple therapy de-escalation to indacaterol/ glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. American Journal of Respiratory and Critical Care Medicine 2018;198(3):329-39. - PubMed
Dransfield 2020 {published data only}
EUCTR2017‐004369‐29‐NL {published data only}
-
- EUCTR2017-004369-29-NL. Single inhaler triple therapy (fluticasone furoate + vilanterol trifenatate + umeclidinium bromide) (inhaled) – COPD. https://www.clinicaltrialsregister.eu/ctr-search/trial/2017-004369-29/re... 2018.
EUCTR2018‐001704‐10‐NL {published data only}
-
- EUCTR2018-001704-10-NL. Phase 3b study to evaluate the effects of budesonide/glycopyrronium/formoterol fuumarate and glycopyrronium/formoterol fumarate on specific image based airway volumes and resistance in subjects with chronic obstructive pulmonary disease (COPD). https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02068001/... 2018.
EUCTR2019‐003351‐11‐NL {published data only}
-
- EUCTR2019-003351-11-NL. Study to the effects of using "triple therapy" on the health status of COPD patients with characteristics of asthma. https://www.clinicaltrialsregister.eu/ctr-search/search?query=2019-00335... 2019.
Ferguson 2020 {published data only}
-
- Ferguson G, Martinez F, Rabe K, Ballal S, Darken P, Aurivillius M, et al. Benefits of budesonide-containing therapies on reducing lung function decline in patients with COPD in the ETHOS study. Chest 2020;158(4 Suppl):A1656-A1659.
Gosden 2018 {published data only}
-
- Gosden TB, Pitcher A, Nour N, Madoni A, Friggi E. A cost-effectiveness analysis of a first-in-class, triple fixed dose combination therapy against LABA/LAMA therapy in moderate-severe COPD. Value in Health 2018;21(Suppl 1):S235.
Halpin 2019 {published data only}
-
- Halpin DMG, Bardsley S, Criner G, Dransfield M, Han MK, Jones CE, et al. The IMPACT trial: single inhaler triple therapy fluticasone furoate/umeclidinium/vilanterol versus fluticasone furoate/vilanterol and umeclidinium/vilanterol in patients with COPD: analysis according to smoking status. American Journal of Respiratory and Critical Care Medicine 2019;199(9):A3339.
Halpin 2020 {published data only}
Han 2020 {published data only}
-
- Han MK, Criner GJ, Dransfield MT, Halpin DMG, Jones CE, Kilbride S, et al. The effect of inhaled corticosteroid withdrawal and baseline inhaled treatment on exacerbations in the IMPACT study. A randomized, double-blind, multicenter clinical trial. American Journal of Respiratory and Critical Care Medicine 2020;202(9):1237-43. - PMC - PubMed
Hara 2007 {published data only}
-
- Hara K, Kurashima K, Tokunaga D, Ueno M, Aoyagi K, Isobe Z, et al. Single blind comparison of tiotropium and salmeterol plus fluticasone propionate of treatment in patients with chronic obstructive pulmonary disease (COPD) [Abstract]. In: American Thoracic Society International Conference; 2007 May 18-23; San Francisco. San Francisco, California, USA, 2007:Poster #A1.
Harada 2020 {published data only}
-
- Harada N, Otsuka K, Horiuchi A, Shinka Y, Yamamura H, Miyao N. Triple therapy with fluticasone furoate/umeclidinium/vilanterol compared with dual bronchodilation or triple therapy with inhaled corticosteroids/dual bronchodilation in patients with chronic obstructive pulmonary disease. European Respiratory Journal 2020;56(suppl 64):3276.
Hovelmann 2018 {published data only}
-
- Hovelmann R, Gessner C. TriOptimize: a prospective non-interventional trial to document potential optimization of health related quality of life in COPD patients prescribed a fixed vs. free LAMA/LABA/ICS triple therapy. Pneumologie 2018;72:S01. [DOI: 10.1055/s-0037-1619366] - DOI
Ichinose 2019 {published data only}
-
- Ichinose M, Fukushima Y, Inoue Y, Hataji O, Ferguson GT, Rabe KF, et al. Long-term safety and efficacy of budesonide/ glycopyrrolate/formoterol fumarate metered dose inhaler formulated using co-suspension delivery technology in Japanese patients with COPD. International Journal of Chronic Obstructive Pulmonary Disease 2019;14:2993-3002. - PMC - PubMed
James 2013 {published data only (unpublished sought but not used)}
-
- James SP, Paul D, Prabhu D, Venugopal KP. A comparative study on inhaled corticosteroids versus placebo in management of chronic obstructive pulmonary disease. Lung India 2013;30(Suppl 1):S29.
Kato 2019 {published data only}
-
- Kato M, Tomii K, Hashimoto K, Nezu Y, Ishii T, Jones CE, et al. The IMPACT study – single inhaler triple therapy (FF/UMEC/VI) versus FF/VI and UMEC/VI in patients with COPD: efficacy and safety in a Japanese population. International Journal of Chronic Obstructive Pulmonary Disease 2019;14:2849-2861. - PMC - PubMed
Kerwin 2019 {published data only}
-
- Kerwin EM, Ferguson GT, Mo M, DeAngelis K, Dorinsky P. Bone mineral density and ocular safety after 52 weeks' treatment with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler (BGF MDI) using co-suspension delivery technology in COPD. American Journal of Respiratory and Critical Care Medicine 2019;199:A3319.
Khan 2018 {published data only}
-
- Khan PA, Sujala A, Sarah Nousheen BB, Fatima AF, Ala HT, Pallavi Reddy ABT. A comparative evaluation of the efficacy of triple drug therapy with dual drug therapy in COPD patients. International Journal of Pharmacy and Pharmaceutical Sciences 2018;10(4):105-9.
Krishnan 2018 {published data only}
-
- Krishnan J, Zappetti D. IMPACT Trial: triple versus dual therapy for COPD results in decreased rate of exacerbations. Clinical Pulmonary Medicine 2018;25(5):195-6.
Lipari 2017 {published data only}
-
- Lipari M, Wilhelm S, Kale-Pradhan P. Triple versus dual therapy in COPD: a meta-analysis. Pharmacotherapy 2017;32(12):e236. - PubMed
Lipson 2019a {published data only}
-
- Lipson DA, Criner G, Dransfield MT, Halpin DMG, Han MK, Jones CE, et al. The IMPACT trial: single inhaler triple therapy fluticasone furoate/umeclidinium/vilanterol versus fluticasone furoate/vilanterol and umeclidinium/vilanterol in patients with COPD: results on cardiovascular safety. American Journal of Respiratory and Critical Care Medicine 2019;199:A3334.
Lipson 2019b {published data only}
-
- Lipson DA, Criner G, Dransfield M, Halpin D, Han M, Elaine Jones C, et al. The IMPACT Trial: single inhaler triple therapy vs dual therapies: efficacy across multiple COPD endpoints over time. European Respiratory Journal 2019;54:PA2482. [DOI: 10.1183/13993003.congress-2019.PA2482] - DOI
Magnussen 2014 {published data only}
-
- Magnussen H, Disse B, Rodriguez-Roisin R, Kirsten A, Watz H, Tetzlaff K, et al. Exacerbation risk is not worse when ICS are withdrawn in a stepwise manner in severe to very severe COPD patients receiving LAMA+LABA: The WISDOM study. Chest 2014;146(4):63A.
-
- Magnussen H, Disse B, Rodriguez-Roisin R, Kirsten A, Watz H, Tetzlaff K, et al. The impact of stepwise withdrawal of ICS on FEV1, mMRC, and SQRQ in severe to very severe COPD patients treated with LAMA+LABA: The WISDOM study. Chest 2014;146(4):76A.
-
- Magnussen H, Disse B, Rodriguez-Roisin R, Kirsten A, Watz H, Tetzlaff K, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. New England Journal of Medicine 2014;371(14):1285-94. - PubMed
Mammen 2021 {published data only}
-
- Mammen MJ, Carr TF, Criner GJ, Dransfield MT, Halpin DMG, Han MK, et al. All-cause mortality by subgroup in patients with chronic obstructive pulmonary disease: post hoc analysis of the impact trial. American Journal of Respiratory and Critical Care Medicine 2021;203:A2241.
Martinez 2020 {published data only}
-
- Martinez FJ, Rabe KF, Ferguson GT, Wedzicha JA, Singh D, Wang C, et al. Reduced all-cause mortality in the ETHOS trial of budesonide/glycopyrrolate/formoterol for COPD: a randomized, double-blind, multi-center parallel-group study. American Journal of Respiratory and Critical Care Medicine 2020;203(5):553-64. - PMC - PubMed
Masayuki 2018 {published data only}
-
- Masayuki H. Clinical usefulness of once-daily triple therapy, ciclesonide and tiotropium bromide/olodaterol combination inhaler for COPD patients. American Journal of Respiratory and Critical Care Medicine 2018;197:A4770.
Mehta 2018 {published data only}
-
- Mehta R, Farrell C, Kilbride S, Zhu C, Brooks J, Barnhart F, et al. Population pharmacokinetic analysis of single inhaler triple therapy (ICS/LAMA/LABA) versus dual therapy (LAMA/LABA AND ICS/LABA) in patients with symptomatic COPD: combined results from three phase iii trials. American Journal of Respiratory and Critical Care Medicine 2018;197:A3029.
Montuschi 2016 {published data only}
-
- Montuschi P, Malerba M, Macis G, Mores N, Santini G. Triple inhaled therapy for chronic obstructive pulmonary disease. Drug Discovery Today 2016;21(11):1820-7. - PubMed
NCT01911364 {published data only}
-
- NCT01911364. Efficacy of fixed combination of beclometasone + formoterol + glycopyrrolate in chronic obstructive pulmonary disease [A 52-wk randomized double blind parallel trial: combination of beclometasone+formoterol+glycopyrrolate vs tiotropium and vs combination of beclometasone+formoterol and tiotropium in patients with chronic obstructive pulmonary disease]. clinicaltrials.gov/ct2/show/NCT01911364 (first received 30 July 2013).
NCT02729051 {published data only}
-
- NCT02729051. Comparative study of fluticasone furoate(FF)/umeclidinium bromide (UMEC)/ vilanterol (VI) closed therapy versus FF/VI plus UMEC open therapy in subjects with chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/NCT02729051 (first received 6 April 2016).
NCT02731846 {published data only}
-
- NCT02731846. A study comparing the closed triple therapy, open triple therapy and a dual therapy for effect on lung function in subjects with chronic obstructive pulmonary disease (COPD) [A phase III, 4-week, randomized, double-blind study to compare 'closed' triple therapy (FF/UMEC/VI), 'open' triple therapy (FF/VI + UMEC) and dual therapy (FF/VI) in subjects with chronic obstructive pulmonary disease (COPD)]. clinicaltrials.gov/ct2/show/NCT02731846 (first received 8 April 2016).
NCT03478683 {published data only}
-
- NCT03478683. A randomized study, comparing fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) single inhaler triple therapy, versus multiple inhaler therapy (budesonide/formoterol plus tiotropium) in subjects with chronic obstructive pulmonary disease (COPD) [A phase IV, 12-week, randomised, double-blind, triple dummy study to compare single inhaler triple therapy, fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) with multiple inhaler therapy (budesonide/formoterol plus tiotropium) based on lung function and symptoms in participants with chronic obstructive pulmonary disease]. clinicaltrials.gov/ct2/show/NCT03478683 (first received 27 March 2018).
NCT04320342 {published data only}
-
- NCT04320342. A phase III study comparing efficacy, safety and tolerability of the fixed dose triple combination CHF 5993 with the fixed dose dual combination CHF 1535 in subjects With COPD. https://clinicaltrials.gov/ct2/show/NCT04320342 (first received 23 March 2020).
NCT04675463 {published data only}
-
- NCT04675463. The effects of inhaled budesonide-formoterol-glycopyrronium in moderate-to-severe COPD. https://clinicaltrials.gov/ct2/show/NCT04675463 (first received 14 December 2020).
NCT04923347 {published data only}
-
- NCT04923347. A study to evaluate the safety and efficacy of fluticasone furoate (FF)/umeclidinium(UMEC)/vilanterol (VI) in participants with chronic obstructive pulmonary disease (COPD). https://clinicaltrials.gov/ct2/show/NCT04923347 (first received 6 June 2021).
Palli 2019 {published data only}
-
- Palli SR, Buikema AR, DuCharme M, Frazer M, Kaila S, Juday T. Costs, exacerbations and pneumonia after initiating combination tiotropium olodaterol versus triple therapy for chronic obstructive pulmonary disease. Journal of Comparative Effectiveness Research 2019;8(15):1299-316. - PubMed
Scuri 2018 {published data only}
-
- Scuri M, Fabbri LM, Singh D, Roche N, Corradi M, Alessandro G, et al. Reduction in fatal events with ICS-containing medications: results of safety pooled analysis from the TRILOGY, TRINITY and TRIBUTE studies. American Journal of Respiratory and Critical Care Medicine 2018;197:A7725.
Singh 2016 {published data only}
-
- Singh D, Papi A, Corradi M, Pavlisova I, Montagna I, Francisco C, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting beta2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet 2016;388(10048):963-73. - PubMed
Singh 2018 {published data only}
-
- Singh D. Single inhaler triple therapy with extrafine beclomethasone, formoterol, and glycopyrronium for the treatment of chronic obstructive pulmonary disease. Expert Opinion on Pharmacotherapy 2018;19(11):1-9. - PubMed
Stiegler 2019 {published data only}
-
- Stiegler MA, Thomashow B, Criner GJ, Dransfield MT, Halpin DMG, Han MK, et al. COPD assessment test (CAT) score is associated with risk of future exacerbation: an analysis from the IMPACT trial. American Journal of Respiratory and Critical Care Medicine 2019;199:A1591.
van den Berge 2020 {published data only}
-
- den Berge M, De Backer J, Van Holsbeke C, De Backer W, Trivedi R, Jenkins M, et al. Functional respiratory imaging assessment of budesonide/glycopyrrolate/formoterol fumarate and glycopyrrolate/formoterol fumarate metered dose inhalers in patients with COPD. Chest 2020;158(4 Suppl):A1782-A1783.
van den Berge 2021 {published data only}
-
- den Berge M, De Backer J, Van Holsbeke C, De Backer W, Trivedi R, Jenkins M, et al. Functional respiratory imaging assessment of budesonide/glycopyrrolate/formoterol fumarate and glycopyrrolate/formoterol fumarate metered dose inhalers in patients with COPD: the value of inhaled corticosteroids. Respiratory Research 2021;22(1):191. - PMC - PubMed
Additional references
Baraldo 2012
-
- Baraldo S, Turato G, Saetta M. Pathophysiology of the small airways in chronic obstructive pulmonary disease. Respiration 2012;84:89-97. - PubMed
Bateman 2014
-
- Bateman ED, Mahler DA, Vogelmeier CF, Wedzicha JA, Patalano F, Banerji D. Recent advances in COPD disease management with fixed-dose long-acting combination therapies. Expert Review of Respiratory Medicine 2014;8(3):357-79. - PubMed
Beeh 2021
-
- Beeh KM, Kuna P, Corradi M, Viaud I, Guasconi A, Georges G. Comparison of dry-powder inhaler and pressurized metered-dose inhaler formulations of extrafine beclomethasone dipropionate/formoterol fumarate/glycopyrronium in patients with COPD: The TRI-D randomized controlled trial. International Journal of Chronic Obstructive Pulmonary Disease 2021;16:79-89. - PMC - PubMed
Buist 2007
-
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD study): a population-based prevalence study. Lancet 2007;370(9589):741-50. - PubMed
COPDX 2019
-
- Yang IA, Brown JL, George J, Jenkins S, McDonald CF, McDonald V, et al. Australian and New Zealand Guidelines for the Management of Chronic Obstructive Pulmonary Disease 2019 Version 2.58, June 2019. copdx.org.au/wp-content/uploads/2019/09/COPDX-V2-58-June-2019-FINAL2.pdf (accessed 3 August 2016).
de Miguel‐Díez 2014
-
- Miguel-Díez J, Jiménez-García R. Considerations for new dual-acting bronchodilator treatments for chronic obstructive pulmonary disease. Expert Opinion on Investigational Drugs 2014;23(4):453-6. - PubMed
Farne 2015
-
- Farne HA, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2015, Issue 10. Art. No: CD008989. [DOI: 10.1002/14651858.CD008989.pub3] - DOI - PubMed
Gaebel 2011
Geake 2015
-
- Geake JB, Dabscheck EJ, Wood-Baker R, Cates CJ. Indacaterol, a once-daily beta2-agonist, versus twice-daily beta2-agonists or placebo for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2015, Issue 1. Art. No: CD010139. [DOI: 10.1002/14651858.CD010139.pub2] - DOI - PMC - PubMed
GOLD 2023
-
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (2023 report). https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2... 2023.
Halpin 2012
Hartl 2020
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2022
-
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.3.
Karner 2011
Karner 2012
-
- Karner C, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 4. Art. No: CD008989. [DOI: 10.1002/14651858.CD008989.pub2] - DOI - PMC - PubMed
Kew 2013
Kew 2014a
Kew 2014b
Lai 2019
-
- Lai CC, Chen CH, Lin CYH, Wang CY, Wang YH. The effects of single inhaler triple therapy vs single inhaler dual therapy or separate triple therapy for the management of chronic obstructive pulmonary disease: a systematic review and meta-analysis of randomized controlled trials. International Journal of COPD 2019;14:1539-48. - PMC - PubMed
Lee 2009
-
- Lee J-H, Lee YK, Kim E-K, Kim T-H, Huh JW, Kim WJ, et al. Responses to inhaled long-acting beta-agonist and corticosteroid according to COPD subtype. Respiratory Medicine 2009;104(4):542-9. - PubMed
Leuppi 2005
-
- Leuppi JD, Tandjung R, Anderson SD, Stolz D, Brutsche MH, Bingisser R, et al. Prediction of treatment-response to inhaled corticosteroids by mannitol-challenge test in COPD. A proof of concept. Pulmonary Pharmacology and Therapeutics 2005;18(2):83-8. - PubMed
Miravitlles 2011
-
- Miravitlles M. Arguments in favor of inhaled corticosteroids in COPD by phenotype instead of by severity. Archivos de Bronconeumologia 2011;47(6):271-3. - PubMed
Montuschi 2013
-
- Montuschi P, Macagno F, Valente S, Fuso L. Inhaled muscarinic acetylcholine receptor antagonists for treatment of COPD. Current Medicinal Chemistry 2013;20(12):1464-1476. - PubMed
Montuschi 2015
-
- Montuschi P, Ciabattoni G. Bronchodilating drugs for chronic obstructive pulmonary disease: current status and future trends. Journal of Medicinal Chemistry 2015;58(10):4131-4164. - PubMed
Montuschi 2016
-
- Montuschi P, Malerba M, Macis G, Mores N, Santini G. Triple inhaled therapy for chronic obstructive pulmonary disease. Drug Discovery Today 2016;21(11):1820-1827. - PubMed
NICE 2019
-
- National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management NICE guideline [NG115]. www.nice.org.uk/guidance/ng115 (accessed prior to 8 October 2019). - PubMed
Pascoe 2019
-
- Pascoe S, Barnes N, Brusselle G, Compton C, Criner GJ, Dransfield MT, et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: analysis of the IMPACT trial. Lancet Respiratory Medicine 2019;7(9):745-56. [DOI: 10.1016/S2213-2600(19)30190-0] - DOI - PubMed
PlotDigitizer [Computer program]
-
- PlotDigitizer. Porbital, 2022. Available at plotdigitizer.com.
Rennard 2004
-
- Rennard SI, Farmer SG. Exacerbations and progression of disease in asthma and chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society 2004;1(2):88-92. - PubMed
Review Manager 2020 [Computer program]
-
- Review Manager (RevMan). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Rodrigo 2014
-
- Rodrigo GJ, Plaza V. Efficacy and safety of a fixed-dose combination of indacaterol and glycopyrronium for the treatment of COPD: a systematic review. Chest 2014;146(2):309-17. - PubMed
Rücker 2017
Siva 2007
-
- Siva R, Green RH, Brightling CE, Shelley M, Hargadon B, McKenna S, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. European Respiratory Journal 2007;29(5):906-13. - PubMed
Spencer 2011
-
- Spencer S, Karner C, Cates CJ, Evans DJ. Inhaled corticosteroids versus long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 12. Art. No: CD007033. [DOI: 10.1002/14651858.CD007033.pub2] - DOI
van der Molen 2012
van der Valk 2002
-
- Valk P, Monninkhof E, Palen J, Zielhuis G, Herwaarden C. Effect of discontinuation of inhaled corticosteroids in patients with chronic obstructive pulmonary disease: the COPE study. American Journal of Respiratory and Critical Care Medicine 2002;166(10):1358-63. - PubMed
van Geffen 2016
van Geffen 2018
van Geffen 2019
-
- Geffen WH, Slebos DJ, Herth FJ, Kemp SV, Weder W, Shah PL. Surgical and endoscopic interventions that reduce lung volume for emphysema: a systemic review and meta-analysis. The Lancet. Respiratory Medicine 2019;7(4):313-24. [PMID: ] - PubMed
van Geffen 2020
-
- Geffen WH, Carpaij OA, Westbroek LF, Seigers D, Niemeijer A, Vonk JM, et al. Long-acting dual bronchodilator therapy (indacaterol/glycopyrronium) versus nebulized short-acting dual bronchodilator (salbutamol/ipratropium) in chronic obstructive pulmonary disease: a double-blind, randomized, placebo-controlled trial. Respiratory Medicine 2020;171:106064. - PubMed
Vestbo 2011
-
- Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, et al. Changes in forced expiratory volume in 1 second over time in COPD. New England Journal of Medicine 2011;365(13):1184-92. - PubMed
Wang 2005
-
- Wang Q, Bourbeau J. Outcomes and health-related quality of life following hospitalisation for an acute exacerbation of COPD. Respirology 2005;10(3):334-40. - PubMed
Watz 2016
-
- Watz H, Tetzlaff K, Wouters EFM, Kirsten A, Magnussen H, Rodriguez-Roisin R, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respiratory Medicine 2016;4(5):390-398. - PubMed
Wedzicha 2013
WHO 2012
-
- World Health Organization. WHO Global Report: mortality attributable to tobacco. whqlibdoc.who.int/publications/2012/9789241564434_eng.pdf (accessed 11 December 2014).
Yang 2012
Zayed 2018
-
- Zayed Y, Barbarawi M, Kheiri B, Haykal T, Chahine A, Rashdan L, et al. Triple versus dual inhaler therapy in moderate-to-severe COPD: a systematic review and meta-analysis of randomized controlled trials. Clinical Respiratory Journal 2018;13(7):413-28. - PubMed
References to other published versions of this review
Tan 2016
-
- Tan DJ, White CJ, Walters JAE, Walters EH. Inhaled corticosteroids with combination inhaled long‐acting beta2‐agonists and long‐acting muscarinic antagonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2016, Issue 11. Art. No: CD011600. [DOI: 10.1002/14651858.CD011600.pub2] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

