Anti-CMV therapy, what next? A systematic review
- PMID: 38053548
- PMCID: PMC10694278
- DOI: 10.3389/fmicb.2023.1321116
Anti-CMV therapy, what next? A systematic review
Abstract
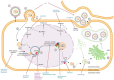
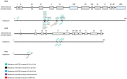
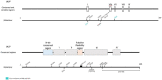
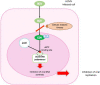
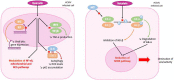
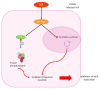
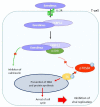
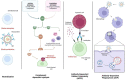
Human cytomegalovirus (HCMV) is one of the main causes of serious complications in immunocompromised patients and after congenital infection. There are currently drugs available to treat HCMV infection, targeting viral polymerase, whose use is complicated by toxicity and the emergence of resistance. Maribavir and letermovir are the latest antivirals to have been developed with other targets. The approval of letermovir represents an important innovation for CMV prevention in hematopoietic stem cell transplant recipients, whereas maribavir allowed improving the management of refractory or resistant infections in transplant recipients. However, in case of multidrug resistance or for the prevention and treatment of congenital CMV infection, finding new antivirals or molecules able to inhibit CMV replication with the lowest toxicity remains a critical need. This review presents a range of molecules known to be effective against HCMV. Molecules with a direct action against HCMV include brincidofovir, cyclopropavir and anti-terminase benzimidazole analogs. Artemisinin derivatives, quercetin and baicalein, and anti-cyclooxygenase-2 are derived from natural molecules and are generally used for different indications. Although they have demonstrated indirect anti-CMV activity, few clinical studies were performed with these compounds. Immunomodulating molecules such as leflunomide and everolimus have also demonstrated indirect antiviral activity against HCMV and could be an interesting complement to antiviral therapy. The efficacy of anti-CMV immunoglobulins are discussed in CMV congenital infection and in association with direct antiviral therapy in heart transplanted patients. All molecules are described, with their mode of action against HCMV, preclinical tests, clinical studies and possible resistance. All these molecules have shown anti-HCMV potential as monotherapy or in combination with others. These new approaches could be interesting to validate in clinical trials.
Keywords: cytomegalovirus; direct antivirals; immunoglobulins; immunomodulatory molecules; indirect antivirals; letermovir; maribavir.
Copyright © 2023 Gourin, Alain and Hantz.
Conflict of interest statement
SA is an expert for Takeda, MSD, Merck and Biotest with no personal link. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Current Perspectives on Letermovir and Maribavir for the Management of Cytomegalovirus Infection in Solid Organ Transplant Recipients.Drug Des Devel Ther. 2024 Sep 6;18:3987-4001. doi: 10.2147/DDDT.S265644. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39258274 Free PMC article. Review.
-
Clinical development of letermovir and maribavir: Overview of human cytomegalovirus drug resistance.Antiviral Res. 2019 Mar;163:91-105. doi: 10.1016/j.antiviral.2019.01.011. Epub 2019 Jan 25. Antiviral Res. 2019. PMID: 30690043 Review.
-
In vitro drug combination studies of Letermovir (AIC246, MK-8228) with approved anti-human cytomegalovirus (HCMV) and anti-HIV compounds in inhibition of HCMV and HIV replication.Antimicrob Agents Chemother. 2015;59(6):3140-8. doi: 10.1128/AAC.00114-15. Epub 2015 Mar 16. Antimicrob Agents Chemother. 2015. PMID: 25779572 Free PMC article.
-
Letermovir for the prevention of cytomegalovirus infection in adult cytomegalovirus-seropositive hematopoietic stem cell transplant recipients.Expert Rev Clin Pharmacol. 2018 Oct;11(10):931-941. doi: 10.1080/17512433.2018.1500897. Epub 2018 Sep 18. Expert Rev Clin Pharmacol. 2018. PMID: 30004790 Review.
-
Investigational Antiviral Therapy Models for the Prevention and Treatment of Congenital Cytomegalovirus Infection during Pregnancy.Antimicrob Agents Chemother. 2020 Dec 16;65(1):e01627-20. doi: 10.1128/AAC.01627-20. Print 2020 Dec 16. Antimicrob Agents Chemother. 2020. PMID: 33077661 Free PMC article.
Cited by
-
Management of Cytomegalovirus Infections in the Era of the Novel Antiviral Players, Letermovir and Maribavir.Infect Dis Rep. 2024 Jan 18;16(1):65-82. doi: 10.3390/idr16010005. Infect Dis Rep. 2024. PMID: 38247977 Free PMC article. Review.
-
'Getting Better'-Is It a Feasible Strategy of Broad Pan-Antiherpesviral Drug Targeting by Using the Nuclear Egress-Directed Mechanism?Int J Mol Sci. 2024 Feb 29;25(5):2823. doi: 10.3390/ijms25052823. Int J Mol Sci. 2024. PMID: 38474070 Free PMC article. Review.
-
Understanding the Cytomegalovirus Cyclin-Dependent Kinase Ortholog pUL97 as a Multifaceted Regulator and an Antiviral Drug Target.Cells. 2024 Aug 13;13(16):1338. doi: 10.3390/cells13161338. Cells. 2024. PMID: 39195228 Free PMC article. Review.
-
Treatment outcomes in cytomegalovirus anterior uveitis.Sci Rep. 2024 Jul 2;14(1):15210. doi: 10.1038/s41598-024-66224-5. Sci Rep. 2024. PMID: 38956212 Free PMC article.
-
ATRX restricts Human Cytomegalovirus (HCMV) viral DNA replication through heterochromatinization and minimizes unpackaged viral genomes.PLoS Pathog. 2024 Sep 5;20(9):e1012516. doi: 10.1371/journal.ppat.1012516. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39236084 Free PMC article.
References
-
- Alain S., Feghoul L., Girault S., Lepiller Q., Frobert E., Michonneau D., et al. . (2020). Letermovir breakthroughs during the French named patient Programme: interest of monitoring blood concentration in clinical practice. J. Antimicrob. Chemother. 75, 2253–2257. doi: 10.1093/jac/dkaa135, PMID: - DOI - PubMed
-
- Aldern K. A., Ciesla S. L., Winegarden K. L., Hostetler K. Y. (2003). Increased antiviral activity of 1-O-hexadecyloxypropyl-[2-(14)C]cidofovir in MRC-5 human lung fibroblasts is explained by unique cellular uptake and metabolism. Mol. Pharmacol. 63, 678–681. doi: 10.1124/mol.63.3.678 - DOI - PubMed
-
- Alsuliman T., Kitel C., Dulery R., Guillaume T., Larosa F., Cornillon J., et al. . (2018). Cytotect®CP as salvage therapy in patients with CMV infection following allogeneic hematopoietic cell transplantation: a multicenter retrospective study. Bone Marrow Transplant. 53, 1328–1335. doi: 10.1038/s41409-018-0166-9 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

