Gut flora in multiple sclerosis: implications for pathogenesis and treatment
- PMID: 38051890
- PMCID: PMC10883522
- DOI: 10.4103/1673-5374.387974
Gut flora in multiple sclerosis: implications for pathogenesis and treatment
Abstract
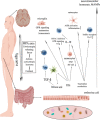
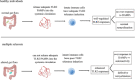
Multiple sclerosis is an inflammatory disorder characterized by inflammation, demyelination, and neurodegeneration in the central nervous system. Although current first-line therapies can help manage symptoms and slow down disease progression, there is no cure for multiple sclerosis. The gut-brain axis refers to complex communications between the gut flora and the immune, nervous, and endocrine systems, which bridges the functions of the gut and the brain. Disruptions in the gut flora, termed dysbiosis, can lead to systemic inflammation, leaky gut syndrome, and increased susceptibility to infections. The pathogenesis of multiple sclerosis involves a combination of genetic and environmental factors, and gut flora may play a pivotal role in regulating immune responses related to multiple sclerosis. To develop more effective therapies for multiple sclerosis, we should further uncover the disease processes involved in multiple sclerosis and gain a better understanding of the gut-brain axis. This review provides an overview of the role of the gut flora in multiple sclerosis.
Copyright © 2024 Copyright: © 2024 Neural Regeneration Research.
Conflict of interest statement
Figures



Similar articles
-
The Gut-Brain Axis in Multiple Sclerosis. Is Its Dysfunction a Pathological Trigger or a Consequence of the Disease?Front Immunol. 2021 Sep 21;12:718220. doi: 10.3389/fimmu.2021.718220. eCollection 2021. Front Immunol. 2021. PMID: 34621267 Free PMC article. Review.
-
The Gut-CNS Axis in Multiple Sclerosis.Trends Neurosci. 2020 Aug;43(8):622-634. doi: 10.1016/j.tins.2020.06.002. Epub 2020 Jul 7. Trends Neurosci. 2020. PMID: 32650957 Free PMC article. Review.
-
Gut Microbiota Interact With the Brain Through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging.Front Immunol. 2022 Apr 7;13:796288. doi: 10.3389/fimmu.2022.796288. eCollection 2022. Front Immunol. 2022. PMID: 35464431 Free PMC article. Review.
-
Understanding the Gut-Brain Axis and Its Therapeutic Implications for Neurodegenerative Disorders.Nutrients. 2023 Oct 31;15(21):4631. doi: 10.3390/nu15214631. Nutrients. 2023. PMID: 37960284 Free PMC article. Review.
-
Pediatric multiple sclerosis: an integrated outlook at the interplay between genetics, environment and brain-gut dysbiosis.AIMS Neurosci. 2023 Aug 23;10(3):232-251. doi: 10.3934/Neuroscience.2023018. eCollection 2023. AIMS Neurosci. 2023. PMID: 37841344 Free PMC article. Review.
Cited by
-
Isolation and identification of probiotic Bacillus subtilis AJQ03 from the intestinal tract of Anguilla japonica (Japanese eel).Front Microbiol. 2024 Oct 30;15:1446299. doi: 10.3389/fmicb.2024.1446299. eCollection 2024. Front Microbiol. 2024. PMID: 39539702 Free PMC article.
-
Relationship between gut microbiota and multiple sclerosis: a scientometric visual analysis from 2010 to 2023.Front Immunol. 2024 Aug 19;15:1451742. doi: 10.3389/fimmu.2024.1451742. eCollection 2024. Front Immunol. 2024. PMID: 39224586 Free PMC article.
-
Infiltration by monocytes of the central nervous system and its role in multiple sclerosis: reflections on therapeutic strategies.Neural Regen Res. 2025 Mar 1;20(3):779-793. doi: 10.4103/NRR.NRR-D-23-01508. Epub 2024 Apr 3. Neural Regen Res. 2025. PMID: 38886942 Free PMC article.
References
-
- Adkins Y, Soulika AM, Mackey B, Kelley DS. Docosahexaenoic acid (22:6n-3) Ameliorated the onset and severity of experimental autoimmune encephalomyelitis in mice. Lipids. 2019;54:13–23. - PubMed
-
- Agirman G, Yu KB, Hsiao EY. Signaling inflammation across the gut-brain axis. Science. 2021;374:1087–1092. - PubMed
-
- Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23:716–724. - PubMed
-
- Anderson G, Maes M. Gut dysbiosis dysregulates central and systemic homeostasis via suboptimal mitochondrial function: assessment, treatment and classification implications. Curr Top Med Chem. 2020;20:524–539. - PubMed
LinkOut - more resources
Full Text Sources

