This is a preprint.
Progressive degeneration in a new Drosophila model of Spinocerebellar Ataxia type 7
- PMID: 38045332
- PMCID: PMC10690306
- DOI: 10.21203/rs.3.rs-3592641/v1
Progressive degeneration in a new Drosophila model of Spinocerebellar Ataxia type 7
Update in
-
Progressive degeneration in a new Drosophila model of spinocerebellar ataxia type 7.Sci Rep. 2024 Jun 21;14(1):14332. doi: 10.1038/s41598-024-65172-4. Sci Rep. 2024. PMID: 38906973 Free PMC article.
Abstract
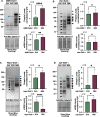
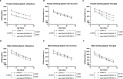
Spinocerebellar ataxia type 7 (SCA7) is a progressive neurodegenerative disorder resulting from abnormal expansion of polyglutamine (polyQ) in its disease protein, ataxin-7 (ATXN7). ATXN7 is part of Spt-Ada-Gcn5 acetyltransferase (SAGA), an evolutionarily conserved transcriptional coactivation complex with critical roles in chromatin remodeling, cell signaling, neurodifferentiation, mitochondrial health and autophagy. SCA7 is dominantly inherited and characterized by genetic anticipation and high repeat-length instability. Patients with SCA7 experience progressive ataxia, atrophy, spasticity, and blindness. There is currently no cure for SCA7, and therapies are aimed at alleviating symptoms to increase quality of life. Here, we report novel Drosophila lines of SCA7 with polyQ repeats in wild-type and human disease patient range. We find that ATXN7 expression has age- and polyQ repeat length-dependent reduction in survival and retinal instability, concomitant with increased ATXN7 protein aggregation. These new lines will provide important insight on disease progression that can be used in the future to identify therapeutic targets for SCA7 patients.
Keywords: Aggregation; Polyglutamine; Proteinopathy; Retinal degeneration.
Conflict of interest statement
Competing Interests Statement The authors declare that they do not have any conflicts of interest to disclose.
Figures



Similar articles
-
Progressive degeneration in a new Drosophila model of Spinocerebellar Ataxia type 7.bioRxiv [Preprint]. 2023 Nov 11:2023.11.07.566106. doi: 10.1101/2023.11.07.566106. bioRxiv. 2023. Update in: Sci Rep. 2024 Jun 21;14(1):14332. doi: 10.1038/s41598-024-65172-4. PMID: 37986914 Free PMC article. Updated. Preprint.
-
Progressive degeneration in a new Drosophila model of spinocerebellar ataxia type 7.Sci Rep. 2024 Jun 21;14(1):14332. doi: 10.1038/s41598-024-65172-4. Sci Rep. 2024. PMID: 38906973 Free PMC article.
-
The Molecular Basis of Spinocerebellar Ataxia Type 7.Front Neurosci. 2022 Mar 24;16:818757. doi: 10.3389/fnins.2022.818757. eCollection 2022. Front Neurosci. 2022. PMID: 35401096 Free PMC article. Review.
-
Molecular Targets and Therapeutic Strategies in Spinocerebellar Ataxia Type 7.Neurotherapeutics. 2019 Oct;16(4):1074-1096. doi: 10.1007/s13311-019-00778-5. Neurotherapeutics. 2019. PMID: 31432449 Free PMC article. Review.
-
Pulling complexes out of complex diseases: Spinocerebellar Ataxia 7.Rare Dis. 2014 Apr 14;2:e28859. doi: 10.4161/rdis.28859. eCollection 2014. Rare Dis. 2014. PMID: 25054097 Free PMC article.
References
-
- Todi S. V., Williams A. & Paulson H. in Molecular Neurology (ed Waxman S. G) Ch. 17, 257–276 (Academic Press, 2007).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

