Production of Mono-Hydroxylated Derivatives of Terpinen-4-ol by Bacterial CYP102A1 Enzymes
- PMID: 38044690
- PMCID: PMC11016761
- DOI: 10.4014/jmb.2310.10018
Production of Mono-Hydroxylated Derivatives of Terpinen-4-ol by Bacterial CYP102A1 Enzymes
Abstract
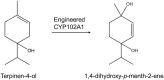
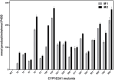
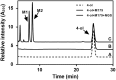
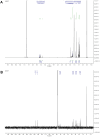
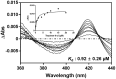
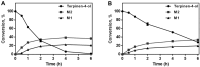
CYP102A1 from Bacillus megaterium is an important enzyme in biotechnology, because engineered CYP102A1 enzymes can react with diverse substrates and produce human cytochrome P450-like metabolites. Therefore, CYP102A1 can be applied to drug metabolite production. Terpinen-4-ol is a cyclic monoterpene and the primary component of essential tea tree oil. Terpinen-4-ol was known for therapeutic effects, including antibacterial, antifungal, antiviral, and anti-inflammatory. Because terpenes are natural compounds, examining novel terpenes and investigating the therapeutic effects of terpenes represent responses to social demands for eco-friendly compounds. In this study, we investigated the catalytic activity of engineered CYP102A1 on terpinen-4-ol. Among CYP102A1 mutants tested here, the R47L/F81I/F87V/E143G/L188Q/N213S/E267V mutant showed the highest activity to terpinen-4-ol. Two major metabolites of terpinen-4-ol were generated by engineered CYP102A1. Characterization of major metabolites was confirmed by liquid chromatography-mass spectrometry (LC-MS), gas chromatography-MS, and nuclear magnetic resonance spectroscopy (NMR). Based on the LC-MS results, the difference in mass-to-charge ratio of an ion (m/z) between terpinen-4-ol and its major metabolites was 16. One major metabolite was defined as 1,4-dihydroxy-p-menth-2-ene by NMR. Given these results, we speculate that another major metabolite is also a mono-hydroxylated product. Taken together, we suggest that CYP102A1 can be applied to make novel terpene derivatives.
Keywords: CYP102A1 mutant; Cytochrome P450; carbon hydroxylation; cyclic monoterpene; terpinen-4-ol.
Conflict of interest statement
The authors have no financial conflicts of interest to declare.
Figures

Similar articles
-
Production of derivatives of α-terpineol by bacterial CYP102A1 enzymes.Biotechnol Lett. 2024 Nov 25;47(1):1. doi: 10.1007/s10529-024-03540-w. Biotechnol Lett. 2024. PMID: 39585478
-
Epoxidation of perillyl alcohol by engineered bacterial cytochrome P450 BM3.Enzyme Microb Technol. 2024 Oct;180:110487. doi: 10.1016/j.enzmictec.2024.110487. Epub 2024 Jul 29. Enzyme Microb Technol. 2024. PMID: 39079222
-
Regioselective hydroxylation of omeprazole enantiomers by bacterial CYP102A1 mutants.Drug Metab Dispos. 2014 Sep;42(9):1493-7. doi: 10.1124/dmd.114.058636. Epub 2014 Jul 9. Drug Metab Dispos. 2014. PMID: 25008345
-
The Versatile Biocatalyst of Cytochrome P450 CYP102A1: Structure, Function, and Engineering.Molecules. 2023 Jul 12;28(14):5353. doi: 10.3390/molecules28145353. Molecules. 2023. PMID: 37513226 Free PMC article. Review.
-
P450(BM3) (CYP102A1): connecting the dots.Chem Soc Rev. 2012 Feb 7;41(3):1218-60. doi: 10.1039/c1cs15192d. Epub 2011 Oct 18. Chem Soc Rev. 2012. PMID: 22008827 Review.
References
-
- Schnitzler P, Schön K, Reichling J. Antiviral activity of Australian tea tree oil and eucalyptus oil against herpes simplex virus in cell culture. Pharmazie. 2001;56:343–347. - PubMed

