Enteropathogenic E. coli infection co-elicits lysosomal exocytosis and lytic host cell death
- PMID: 38038448
- PMCID: PMC10746156
- DOI: 10.1128/mbio.01979-23
Enteropathogenic E. coli infection co-elicits lysosomal exocytosis and lytic host cell death
Abstract
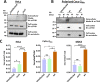
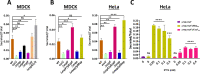
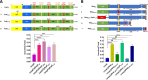
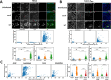
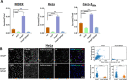
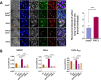
Enteropathogenic Escherichia coli (EPEC) infection is a significant cause of gastroenteritis, mainly in children. Therefore, studying the mechanisms of EPEC infection is an important research theme. EPEC modulates its host cell life by injecting via a type III secretion machinery cell death modulating effector proteins. For instance, while EspF and Map promote mitochondrial cell death, EspZ antagonizes cell death. We show that these effectors also control lysosomal exocytosis, i.e., the trafficking of lysosomes to the host cell plasma membrane. Interestingly, the capacity of these effectors to induce or protect against cell death correlates completely with their ability to induce LE, suggesting that the two processes are interconnected. Modulating host cell death is critical for establishing bacterial attachment to the host and subsequent dissemination. Therefore, exploring the modes of LE involvement in host cell death is crucial for elucidating the mechanisms underlying EPEC infection and disease.
Keywords: EspF; EspZ; Map; cell death; enteropathogenic E. coli; host-pathogen interactions; lysosomal exocytosis; membrane repair; type III secreted effectors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
EspH utilizes phosphoinositide and Rab binding domains to interact with plasma membrane infection sites and Rab GTPases.Gut Microbes. 2024 Jan-Dec;16(1):2400575. doi: 10.1080/19490976.2024.2400575. Epub 2024 Sep 23. Gut Microbes. 2024. PMID: 39312647 Free PMC article.
-
EspZ of enteropathogenic and enterohemorrhagic Escherichia coli regulates type III secretion system protein translocation.mBio. 2012 Oct 2;3(5):e00317-12. doi: 10.1128/mBio.00317-12. Print 2012. mBio. 2012. PMID: 23033475 Free PMC article.
-
The Complete Genome of the Atypical Enteropathogenic Escherichia coli Archetype Isolate E110019 Highlights a Role for Plasmids in Dissemination of the Type III Secreted Effector EspT.Infect Immun. 2019 Sep 19;87(10):e00412-19. doi: 10.1128/IAI.00412-19. Print 2019 Oct. Infect Immun. 2019. PMID: 31358567 Free PMC article.
-
Dynamics of expression, secretion and translocation of type III effectors during enteropathogenic Escherichia coli infection.Curr Opin Microbiol. 2020 Apr;54:67-76. doi: 10.1016/j.mib.2019.12.001. Epub 2020 Feb 12. Curr Opin Microbiol. 2020. PMID: 32058947 Review.
-
Modulation of Host Cell Processes by T3SS Effectors.Curr Top Microbiol Immunol. 2018;416:73-115. doi: 10.1007/82_2018_106. Curr Top Microbiol Immunol. 2018. PMID: 30178263 Review.
Cited by
-
Activation of ARP2/3 and HSP70 Expression by Lipoteichoic Acid: Potential Bidirectional Regulation of Apoptosis in a Mastitis Inflammation Model.Biomolecules. 2024 Jul 25;14(8):901. doi: 10.3390/biom14080901. Biomolecules. 2024. PMID: 39199289 Free PMC article.
-
EspH utilizes phosphoinositide and Rab binding domains to interact with plasma membrane infection sites and Rab GTPases.Gut Microbes. 2024 Jan-Dec;16(1):2400575. doi: 10.1080/19490976.2024.2400575. Epub 2024 Sep 23. Gut Microbes. 2024. PMID: 39312647 Free PMC article.
-
The C-terminal proline-rich repeats of Enteropathogenic E. coli effector EspF are sufficient for the depletion of tight junction membrane proteins and interactions with early and recycling endosomes.Gut Pathog. 2024 Jul 7;16(1):36. doi: 10.1186/s13099-024-00626-8. Gut Pathog. 2024. PMID: 38972985 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

