Effect of standard and physiological cell culture temperatures on in vitro proliferation and differentiation of primary broiler chicken pectoralis major muscle satellite cells
- PMID: 38033332
- PMCID: PMC10687209
- DOI: 10.3389/fphys.2023.1288809
Effect of standard and physiological cell culture temperatures on in vitro proliferation and differentiation of primary broiler chicken pectoralis major muscle satellite cells
Abstract
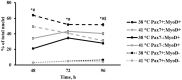
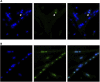
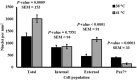
Culture temperatures for broiler chicken cells are largely based on those optimized for mammalian species, although normal broiler body temperature is typically more than 3°C higher. The objective was to evaluate the effects of simulating broiler peripheral muscle temperature, 41°C, compared with standard temperature, 38°C, on the in vitro proliferation and differentiation of primary muscle-specific stem cells (satellite cells; SC) from the pectoralis major (PM) of broiler chickens. Primary SC cultures were isolated from the PM of 18-day-old Ross 708 × Yield Plus male broilers. SC were plated in triplicate, 1.8-cm2, gelatin-coated wells at 40,000 cells per well. Parallel plates were cultured at either 38°C or 41°C in separate incubators. At 48, 72, and 96 h post-plating, the culture wells were fixed and immunofluorescence-stained to determine the expression of the myogenic regulatory factors Pax7 and MyoD as well as evaluated for apoptosis using a TUNEL assay. After 168 h in culture, plates were immunofluorescence-stained to visualize myosin heavy chain and Pax7 expression and determine myotube characteristics and SC fusion. Population doubling times were not impacted by temperature (p ≥ 0.1148), but culturing broiler SC at 41°C for 96 h promoted a more rapid progression through myogenesis, while 38°C maintained primitive populations (p ≤ 0.0029). The proportion of apoptotic cells increased in primary SC cultured at 41°C (p ≤ 0.0273). Culturing at 41°C appeared to negatively impact fusion percentage (p < 0.0001) and tended to result in the formation of thinner myotubes (p = 0.061) without impacting the density of differentiated cells (p = 0.7551). These results indicate that culture temperature alters primary broiler PM SC myogenic kinetics and has important implications for future in vitro work as well as improving our understanding of how thermal manipulation can alter myogenesis patterns during broiler embryonic and post-hatch muscle growth.
Keywords: apoptosis; broiler chicken; cell culture temperature; muscle satellite cell; myogenesis; myogenic regulatory factor.
Copyright © 2023 Gregg, Hutson, Flees, Starkey and Starkey.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Technical note: an optimized method to isolate, purify, and differentiate satellite cells from broiler chicks.J Anim Sci. 2022 Dec 1;100(12):skac342. doi: 10.1093/jas/skac342. J Anim Sci. 2022. PMID: 36271876 Free PMC article.
-
Effect of combined maternal and post-hatch dietary 25-hydroxycholecalciferol supplementation on broiler chicken Pectoralis major muscle growth characteristics and satellite cell mitotic activity.J Anim Sci. 2022 Aug 1;100(8):skac192. doi: 10.1093/jas/skac192. J Anim Sci. 2022. PMID: 35908786 Free PMC article.
-
Effect of Different Basal Culture Media and Sera Type Combinations on Primary Broiler Chicken Muscle Satellite Cell Heterogeneity during Proliferation and Differentiation.Animals (Basel). 2022 May 31;12(11):1425. doi: 10.3390/ani12111425. Animals (Basel). 2022. PMID: 35681889 Free PMC article.
-
Broiler chicken myofiber morphometrics and myogenic stem cell population heterogeneity1,2.Poult Sci. 2019 Sep 1;98(9):4123-4130. doi: 10.3382/ps/pez287. Poult Sci. 2019. PMID: 31144722
-
Timing Is Everything-The High Sensitivity of Avian Satellite Cells to Thermal Conditions During Embryonic and Posthatch Periods.Front Physiol. 2020 Mar 31;11:235. doi: 10.3389/fphys.2020.00235. eCollection 2020. Front Physiol. 2020. PMID: 32300304 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

