BMP2 gene transfer induces pericardial effusion and inflammatory response in the ischemic porcine myocardium
- PMID: 38028463
- PMCID: PMC10655027
- DOI: 10.3389/fcvm.2023.1279613
BMP2 gene transfer induces pericardial effusion and inflammatory response in the ischemic porcine myocardium
Abstract
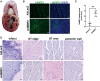
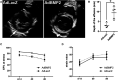
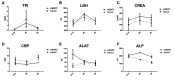
Pro-angiogenic gene therapy is being developed to treat coronary artery disease (CAD). We recently showed that bone morphogenetic protein 2 (BMP2) and vascular endothelial growth factor-A synergistically regulate endothelial cell sprouting in vitro. BMP2 was also shown to induce endocardial angiogenesis in neonatal mice post-myocardial infarction. In this study, we investigated the potential of BMP2 gene transfer to improve cardiomyocyte function and neovessel formation in a pig chronic myocardial infarction model. Ischemia was induced in domestic pigs by placing a bottleneck stent in the proximal part of the left anterior descending artery 14 days before gene transfer. Intramyocardial gene transfers with adenovirus vectors (1 × 1012 viral particles/pig) containing either human BMP2 (AdBMP2) or beta-galactosidase (AdLacZ) control gene were performed using a needle injection catheter. BMP2 transgene expression in the myocardium was detected with immunofluorescence staining in the gene transfer area 6 days after AdBMP2 administration. BMP2 gene transfer did not induce angiogenesis or cardiomyocyte proliferation in the ischemic pig myocardium as determined by the quantitations of CD31 or Ki-67 stainings, respectively. Accordingly, no changes in heart contractility were detected in left ventricular ejection fraction and strain measurements. However, BMP2 gene transfer induced pericardial effusion (AdBMP2: 9.41 ± 3.17 mm; AdLacZ: 3.07 ± 1.33 mm) that was measured by echocardiography. Furthermore, an increase in the number of immune cells and CD3+ T cells was found in the BMP2 gene transfer area. No changes were detected in the clinical chemistry analysis of pig serum or histology of the major organs, implicating that the gene transfer did not induce general toxicity, myocardial injury, or off-target effects. Finally, the levels of fibrosis and cardiomyocyte apoptosis detected by Sirius red or caspase 3 stainings, respectively, remained unaltered between the groups. Our results demonstrate that BMP2 gene transfer causes inflammatory changes and pericardial effusion in the adult ischemic myocardium, which thus does not support its therapeutic use in chronic CAD.
Keywords: BMP2; bone morphogenetic protein 2; coronary artery disease; gene therapy; inflammation; ischemic heart disease; myocardial ischemia; pig myocardium.
© 2023 Pulkkinen, Kivistö-Rahnasto, Korpela, Heikkilä, Järveläinen, Siimes, Kilpeläinen, Laham-Karam, Ylä-Herttuala and Laakkonen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that they were an editorial board member of Frontiers at the time of submission. This had no impact on the peer review process and the final decision.
Figures



Similar articles
-
Citrate-Saline-Formulated mRNA Delivery into the Heart Muscle with an Electromechanical Mapping and Injection Catheter Does Not Lead to Therapeutic Effects in a Porcine Chronic Myocardial Ischemia Model.Hum Gene Ther. 2021 Oct;32(19-20):1295-1307. doi: 10.1089/hum.2021.149. Hum Gene Ther. 2021. PMID: 34494459
-
Safety of direct myocardial administration of an adenovirus vector encoding vascular endothelial growth factor 121.Hum Gene Ther. 1999 May 20;10(8):1331-48. doi: 10.1089/10430349950018012. Hum Gene Ther. 1999. PMID: 10365664
-
Electromagnetic guidance for catheter-based transendocardial injection: a platform for intramyocardial angiogenesis therapy. Results in normal and ischemic porcine models.J Am Coll Cardiol. 2000 Mar 15;35(4):1031-9. doi: 10.1016/s0735-1097(99)00642-7. J Am Coll Cardiol. 2000. PMID: 10732905
-
Granulocyte-colony stimulating factor therapy to induce neovascularization in ischemic heart disease.Dan Med J. 2012 Mar;59(3):B4411. Dan Med J. 2012. PMID: 22381094 Review.
-
Italian Society of Cardiovascular Echography (SIEC) Consensus Conference on the state of the art of contrast echocardiography.Ital Heart J. 2004 Apr;5(4):309-34. Ital Heart J. 2004. PMID: 15185894 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

