Human leukocyte immunoglobulin-like receptors in health and disease
- PMID: 38022598
- PMCID: PMC10679719
- DOI: 10.3389/fimmu.2023.1282874
Human leukocyte immunoglobulin-like receptors in health and disease
Abstract
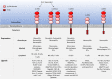
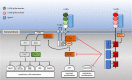
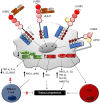
Human leukocyte immunoglobulin (Ig)-like receptors (LILR) are a family of 11 innate immunomodulatory receptors, primarily expressed on lymphoid and myeloid cells. LILRs are either activating (LILRA) or inhibitory (LILRB) depending on their associated signalling domains (D). With the exception of the soluble LILRA3, LILRAs mediate immune activation, while LILRB1-5 primarily inhibit immune responses and mediate tolerance. Abnormal expression and function of LILRs is associated with a range of pathologies, including immune insufficiency (infection and malignancy) and overt immune responses (autoimmunity and alloresponses), suggesting LILRs may be excellent candidates for targeted immunotherapies. This review will discuss the biology and clinical relevance of this extensive family of immune receptors and will summarise the recent developments in targeting LILRs in disease settings, such as cancer, with an update on the clinical trials investigating the therapeutic targeting of these receptors.
Keywords: LILR; autoimmunity; cancer; immune tolerance; immunomodulation; immunotherapy; infection.
Copyright © 2023 Redondo-García, Barritt, Papagregoriou, Yeboah, Frendeus, Cragg and Roghanian.
Conflict of interest statement
BF is an employee of BioInvent International. AR and MSC receive institutional support from BioInvent International. AR acts as a consultant for Epsilogen. MSC has consulted for BioInvent International, Boehringer Ingelheim, GSK, Radiant, iteos, Surrozen, Hanall and Mestag and received research funding from BioInvent International, GSK, UCB and iTeos. He is a member of the GSK Immunology Network. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The Role of Leukocyte Immunoglobulin-Like Receptors Focusing on the Therapeutic Implications of the Subfamily B2.Curr Drug Targets. 2022;23(15):1430-1452. doi: 10.2174/1389450123666220822201605. Curr Drug Targets. 2022. PMID: 36017847
-
LILRB receptor-mediated regulation of myeloid cell maturation and function.Cancer Immunol Immunother. 2017 Aug;66(8):1079-1087. doi: 10.1007/s00262-017-2023-x. Epub 2017 Jun 21. Cancer Immunol Immunother. 2017. PMID: 28638976 Free PMC article. Review.
-
Functional and genetic diversity of leukocyte immunoglobulin-like receptor and implication for disease associations.J Hum Genet. 2015 Nov;60(11):703-8. doi: 10.1038/jhg.2015.64. Epub 2015 Jun 4. J Hum Genet. 2015. PMID: 26040207 Review.
-
Leukocyte Immunoglobulin-Like Receptors in Regulating the Immune Response in Infectious Diseases: A Window of Opportunity to Pathogen Persistence and a Sound Target in Therapeutics.Front Immunol. 2021 Sep 14;12:717998. doi: 10.3389/fimmu.2021.717998. eCollection 2021. Front Immunol. 2021. PMID: 34594332 Free PMC article. Review.
-
The LILR family: modulators of innate and adaptive immune pathways in health and disease.Tissue Antigens. 2004 Sep;64(3):215-25. doi: 10.1111/j.0001-2815.2004.00290.x. Tissue Antigens. 2004. PMID: 15304001 Review.
Cited by
-
IOS-1002, a Stabilized HLA-B57 Open Format, Exerts Potent Anti-Tumor Activity.Cancers (Basel). 2024 Aug 21;16(16):2902. doi: 10.3390/cancers16162902. Cancers (Basel). 2024. PMID: 39199672 Free PMC article.
-
Leukocyte immunoglobulin-like receptor B4: A keystone in immune modulation and therapeutic target in cancer and beyond.Cancer Innov. 2024 Oct 22;3(6):e153. doi: 10.1002/cai2.153. eCollection 2024 Dec. Cancer Innov. 2024. PMID: 39444949 Free PMC article. Review.
-
The Potential Role of the Leucocyte Immunoglobulin-Like Receptors in Kidney Transplant Rejection: A Mini Review.Transpl Int. 2024 Jul 1;37:12995. doi: 10.3389/ti.2024.12995. eCollection 2024. Transpl Int. 2024. PMID: 39010891 Free PMC article. Review.
-
LILRB4 Checkpoint for Immunotherapy: Structure, Mechanism and Disease Targets.Biomolecules. 2024 Feb 4;14(2):187. doi: 10.3390/biom14020187. Biomolecules. 2024. PMID: 38397424 Free PMC article. Review.
-
Inhibitory immune checkpoints suppress the surveillance of senescent cells promoting their accumulation with aging and in age-related diseases.Biogerontology. 2024 Oct;25(5):749-773. doi: 10.1007/s10522-024-10114-w. Epub 2024 Jul 1. Biogerontology. 2024. PMID: 38954358 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

