Hepatocyte-specific regulation of autophagy and inflammasome activation via MyD88 during lethal Ehrlichia infection
- PMID: 38022511
- PMCID: PMC10662044
- DOI: 10.3389/fimmu.2023.1212167
Hepatocyte-specific regulation of autophagy and inflammasome activation via MyD88 during lethal Ehrlichia infection
Abstract
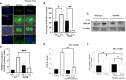
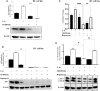
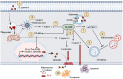
Hepatocytes play a crucial role in host response to infection. Ehrlichia is an obligate intracellular bacterium that causes potentially life-threatening human monocytic ehrlichiosis (HME) characterized by an initial liver injury followed by sepsis and multi-organ failure. We previously showed that infection with highly virulent Ehrlichia japonica (E. japonica) induces liver damage and fatal ehrlichiosis in mice via deleterious MyD88-dependent activation of CASP11 and inhibition of autophagy in macrophage. While macrophages are major target cells for Ehrlichia, the role of hepatocytes (HCs) in ehrlichiosis remains unclear. We investigated here the role of MyD88 signaling in HCs during infection with E. japonica using primary cells from wild-type (WT) and MyD88-/- mice, along with pharmacologic inhibitors of MyD88 in a murine HC cell line. Similar to macrophages, MyD88 signaling in infected HCs led to deleterious CASP11 activation, cleavage of Gasdermin D, secretion of high mobility group box 1, IL-6 production, and inflammatory cell death, while controlling bacterial replication. Unlike macrophages, MyD88 signaling in Ehrlichia-infected HCs attenuated CASP1 activation but activated CASP3. Mechanistically, active CASP1/canonical inflammasome pathway negatively regulated the activation of CASP3 in infected MyD88-/- HCs. Further, MyD88 promoted autophagy induction in HCs, which was surprisingly associated with the activation of the mammalian target of rapamycin complex 1 (mTORC1), a known negative regulator of autophagy. Pharmacologic blocking mTORC1 activation in E. japonica-infected WT, but not infected MyD88-/- HCs, resulted in significant induction of autophagy, suggesting that MyD88 promotes autophagy during Ehrlichia infection not only in an mTORC1-indpenedent manner, but also abrogates mTORC1-mediated inhibition of autophagy in HCs. In conclusion, this study demonstrates that hepatocyte-specific regulation of autophagy and inflammasome pathway via MyD88 is distinct than MyD88 signaling in macrophages during fatal ehrlichiosis. Understanding hepatocyte-specific signaling is critical for the development of new therapeutics against liver-targeting pathogens such as Ehrlichia.
Keywords: Ehrlichia spp.; HMGB1; MyD88; autophagy; inflammasome.
Copyright © 2023 Teymournejad, Sharma, Abdelwahed, Kader, Ahmed, Elkafas and Ismail.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



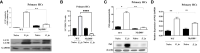
Similar articles
-
Interferon Type I Regulates Inflammasome Activation and High Mobility Group Box 1 Translocation in Hepatocytes During Ehrlichia-Induced Acute Liver Injury.Hepatol Commun. 2020 Sep 27;5(1):33-51. doi: 10.1002/hep4.1608. eCollection 2021 Jan. Hepatol Commun. 2020. PMID: 33437899 Free PMC article.
-
MyD88-dependent inflammasome activation and autophagy inhibition contributes to Ehrlichia-induced liver injury and toxic shock.PLoS Pathog. 2017 Oct 19;13(10):e1006644. doi: 10.1371/journal.ppat.1006644. eCollection 2017 Oct. PLoS Pathog. 2017. PMID: 29049365 Free PMC article.
-
Evasion of host antioxidative response via disruption of NRF2 signaling in fatal Ehrlichia-induced liver injury.PLoS Pathog. 2023 Nov 13;19(11):e1011791. doi: 10.1371/journal.ppat.1011791. eCollection 2023 Nov. PLoS Pathog. 2023. PMID: 37956169 Free PMC article.
-
Non-Canonical Inflammasome Pathway: The Role of Cell Death and Inflammation in Ehrlichiosis.Cells. 2023 Nov 9;12(22):2597. doi: 10.3390/cells12222597. Cells. 2023. PMID: 37998332 Free PMC article. Review.
-
Emerging Roles of Autophagy and Inflammasome in Ehrlichiosis.Front Immunol. 2019 May 8;10:1011. doi: 10.3389/fimmu.2019.01011. eCollection 2019. Front Immunol. 2019. PMID: 31134081 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

