Blood-brain barrier dysfunction and Alzheimer's disease: associations, pathogenic mechanisms, and therapeutic potential
- PMID: 38020775
- PMCID: PMC10679748
- DOI: 10.3389/fnagi.2023.1258640
Blood-brain barrier dysfunction and Alzheimer's disease: associations, pathogenic mechanisms, and therapeutic potential
Abstract
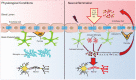
Alzheimer's disease (AD) is a common neurodegenerative disorder characterized by the accumulation of amyloid-beta (Aβ), hyperphosphorylation of tau, and neuroinflammation in the brain. The blood-brain barrier (BBB) limits solutes from circulating blood from entering the brain, which is essential for neuronal functioning. Focusing on BBB function is important for the early detection of AD and in-depth study of AD pathogenic mechanisms. However, the mechanism of BBB alteration in AD is still unclear, which hinders further research on therapeutics that target the BBB to delay the progression of AD. The exact timing of the vascular abnormalities in AD and the complex cause-and-effect relationships remain uncertain. Thus, it is necessary to summarize and emphasize this process. First, in this review, the current evidence for BBB dysfunction in AD is summarized. Then, the interrelationships and pathogenic mechanisms between BBB dysfunction and the risk factors for AD, such as Aβ, tau, neuroinflammation, apolipoprotein E (ApoE) genotype and aging, were analyzed. Finally, we discuss the current status and future directions of therapeutic AD strategies targeting the BBB. We hope that these summaries or reviews will allow readers to better understand the relationship between the BBB and AD.
Keywords: Alzheimer’s disease; aging; apolipoprotein E; blood-brain barrier; neuroinflammation.
Copyright © 2023 Chen, He, Han, Wei and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Discerning the Role of Blood Brain Barrier Dysfunction in Alzheimer's Disease.Aging Dis. 2022 Oct 1;13(5):1391-1404. doi: 10.14336/AD.2022.0130-1. eCollection 2022 Oct 1. Aging Dis. 2022. PMID: 36186141 Free PMC article.
-
Dysfunction of the blood-brain barrier in Alzheimer's disease: Evidence from human studies.Neuropathol Appl Neurobiol. 2022 Apr;48(3):e12782. doi: 10.1111/nan.12782. Epub 2022 Feb 2. Neuropathol Appl Neurobiol. 2022. PMID: 34823269 Review.
-
Regulation of neuroinflammation in Alzheimer's disease via nanoparticle-loaded phytocompounds with anti-inflammatory and autophagy-inducing properties.Phytomedicine. 2024 Jan;122:155150. doi: 10.1016/j.phymed.2023.155150. Epub 2023 Oct 15. Phytomedicine. 2024. PMID: 37944239
-
Decoding Alzheimer's disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies.Prog Neurobiol. 2013 Sep;108:21-43. doi: 10.1016/j.pneurobio.2013.06.004. Epub 2013 Jul 11. Prog Neurobiol. 2013. PMID: 23850509 Review.
-
ApoE4-mediated blood-brain barrier damage in Alzheimer's disease: Progress and prospects.Brain Res Bull. 2023 Jul;199:110670. doi: 10.1016/j.brainresbull.2023.110670. Epub 2023 May 22. Brain Res Bull. 2023. PMID: 37224887 Review.
Cited by
-
Latest Perspectives on Alzheimer's Disease Treatment: The Role of Blood-Brain Barrier and Antioxidant-Based Drug Delivery Systems.Molecules. 2024 Aug 27;29(17):4056. doi: 10.3390/molecules29174056. Molecules. 2024. PMID: 39274904 Free PMC article. Review.
-
Novelties on Neuroinflammation in Alzheimer's Disease-Focus on Gut and Oral Microbiota Involvement.Int J Mol Sci. 2024 Oct 19;25(20):11272. doi: 10.3390/ijms252011272. Int J Mol Sci. 2024. PMID: 39457054 Free PMC article. Review.
-
Circulating small extracellular vesicles in Alzheimer's disease: a case-control study of neuro-inflammation and synaptic dysfunction.BMC Med. 2024 Jun 20;22(1):254. doi: 10.1186/s12916-024-03475-z. BMC Med. 2024. PMID: 38902659 Free PMC article.
-
Risk factors in developing amyloid related imaging abnormalities (ARIA) and clinical implications.Front Neurosci. 2024 Jan 19;18:1326784. doi: 10.3389/fnins.2024.1326784. eCollection 2024. Front Neurosci. 2024. PMID: 38312931 Free PMC article. Review.
-
Therapeutic Targets in Innate Immunity to Tackle Alzheimer's Disease.Cells. 2024 Aug 26;13(17):1426. doi: 10.3390/cells13171426. Cells. 2024. PMID: 39272998 Free PMC article. Review.
References
-
- Akbari E., Asemi Z., Daneshvar Kakhaki R., Bahmani F., Kouchaki E., Tamtaji O. R., et al. . (2016). Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: a randomized, double-blind and controlled trial. Front. Aging Neurosci. 8:256. doi: 10.3389/fnagi.2016.00256 - DOI - PMC - PubMed
-
- Allison Bosworth C. G., Chakhoyan A., Sagare A. P., Nelson A. R., Wang Y., Kisler K., et al. . (2023). Molecular signature and functional properties of human pluripotent stem cell-derived brain pericytes. bioRxiv. doi: 10.1101/2023.06.26.546577 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

