Sulfinamide Formation from the Reaction of Bacillithiol and Nitroxyl
- PMID: 38012810
- PMCID: PMC11229778
- DOI: 10.1021/acschembio.3c00526
Sulfinamide Formation from the Reaction of Bacillithiol and Nitroxyl
Abstract
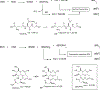
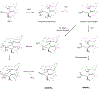
Bacillithiol (BSH) replaces glutathione (GSH) as the most prominent low-molecular-weight thiol in many low G + C gram-positive bacteria. BSH plays roles in metal binding, protein/enzyme regulation, detoxification, redox buffering, and bacterial virulence. Given the small amounts of BSH isolated from natural sources and relatively lengthy chemical syntheses, the reactions of BSH with pertinent reactive oxygen, nitrogen, and sulfur species remain largely unexplored. We prepared BSH and exposed it to nitroxyl (HNO), a reactive nitrogen species that influences bacterial sulfur metabolism. The profile of this reaction was distinct from HNO oxidation of GSH, which yielded mixtures of disulfide and sulfinamide. The reaction of BSH and HNO (generated from Angeli's salt) gives only sulfinamide products, including a newly proposed cyclic sulfinamide. Treatment of a glucosamine-cysteine conjugate, which lacks the malic acid group, with HNO forms disulfide, implicating the malic acid group in sulfinamide formation. This finding supports a mechanism involving the formation of an N-hydroxysulfenamide intermediate that dehydrates to a sulfenium ion that can be trapped by water or internally trapped by an amide nitrogen to give the cyclic sulfinamide. The biological relevance of BSH reactivity toward HNO is provided through in vivo experiments demonstrating that Bacillus subtilis exposed to HNO shows a growth phenotype, and a strain unable to produce BSH shows hypersensitivity toward HNO in minimal medium cultures. Thiol analysis of HNO-exposed cultures shows an overall decrease in reduced BSH levels, which is not accompanied by increased levels of BSSB, supporting a model involving the formation of an oxidized sulfinamide derivative, identified in vivo by high-pressure liquid chromatography/mass spectrometry. Collectively, these findings reveal the unique chemistry and biology of HNO with BSH in bacteria that produce this biothiol.
Figures
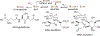

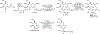
Similar articles
-
Quantitative detection of nitroxyl upon trapping with glutathione and labeling with a specific fluorogenic reagent.Free Radic Biol Med. 2013 Oct;63:476-84. doi: 10.1016/j.freeradbiomed.2013.05.011. Epub 2013 May 16. Free Radic Biol Med. 2013. PMID: 23685286
-
The Role of Bacillithiol in Gram-Positive Firmicutes.Antioxid Redox Signal. 2018 Feb 20;28(6):445-462. doi: 10.1089/ars.2017.7057. Epub 2017 Apr 24. Antioxid Redox Signal. 2018. PMID: 28301954 Free PMC article. Review.
-
Water soluble acyloxy nitroso compounds: HNO release and reactions with heme and thiol containing proteins.J Inorg Biochem. 2013 Jan;118:140-7. doi: 10.1016/j.jinorgbio.2012.07.023. Epub 2012 Oct 6. J Inorg Biochem. 2013. PMID: 23083700 Free PMC article.
-
Nitroxyl (HNO) reacts with molecular oxygen and forms peroxynitrite at physiological pH. Biological Implications.J Biol Chem. 2014 Dec 19;289(51):35570-81. doi: 10.1074/jbc.M114.597740. Epub 2014 Nov 5. J Biol Chem. 2014. PMID: 25378389 Free PMC article.
-
Bacillithiol, a new player in bacterial redox homeostasis.Antioxid Redox Signal. 2011 Jul 1;15(1):123-33. doi: 10.1089/ars.2010.3562. Epub 2010 Dec 17. Antioxid Redox Signal. 2011. PMID: 20712413 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

