Engineered compact pan-neuronal promoter from Alphaherpesvirus LAP2 enhances target gene expression in the mouse brain and reduces tropism in the liver
- PMID: 38012300
- PMCID: PMC11090813
- DOI: 10.1038/s41434-023-00430-0
Engineered compact pan-neuronal promoter from Alphaherpesvirus LAP2 enhances target gene expression in the mouse brain and reduces tropism in the liver
Abstract
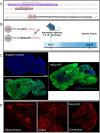
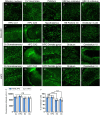
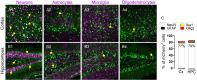
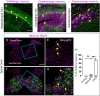
Small promoters capable of driving potent neuron-restricted gene expression are required to support successful brain circuitry and clinical gene therapy studies. However, converting large promoters into functional MiniPromoters, which can be used in vectors with limited capacity, remains challenging. In this study, we describe the generation of a novel version of alphaherpesvirus latency-associated promoter 2 (LAP2), which facilitates precise transgene expression exclusively in the neurons of the mouse brain while minimizing undesired targeting in peripheral tissues. Additionally, we aimed to create a compact neural promoter to facilitate packaging of larger transgenes. Our results revealed that MiniLAP2 (278 bp) drives potent transgene expression in all neurons in the mouse brain, with little to no expression in glial cells. In contrast to the native promoter, MiniLAP2 reduced tropism in the spinal cord and liver. No expression was detected in the kidney or skeletal muscle. In summary, we developed a minimal pan-neuronal promoter that drives specific and robust transgene expression in the mouse brain when delivered intravenously via AAV-PHP.eB vector. The use of this novel MiniPromoter may broaden the range of deliverable therapeutics and improve their safety and efficacy by minimizing the potential for off-target effects.
© 2023. The Author(s).
Conflict of interest statement
The author declares no competing interests.
Figures


Similar articles
-
Local and systemic administration of AAV vectors with alphaherpesvirus latency-associated promoter 2 drives potent transgene expression in mouse liver, kidney, and skeletal muscle.J Virol Methods. 2023 Apr;314:114688. doi: 10.1016/j.jviromet.2023.114688. Epub 2023 Feb 1. J Virol Methods. 2023. PMID: 36736702 Free PMC article.
-
New MiniPromoter Ple345 (NEFL) Drives Strong and Specific Expression in Retinal Ganglion Cells of Mouse and Primate Retina.Hum Gene Ther. 2019 Mar;30(3):257-272. doi: 10.1089/hum.2018.118. Epub 2018 Oct 2. Hum Gene Ther. 2019. PMID: 30062914 Free PMC article.
-
Comparative study of neuron-specific promoters in mouse brain transduced by intravenously administered AAV-PHP.eB.Neurosci Lett. 2021 Jun 21;756:135956. doi: 10.1016/j.neulet.2021.135956. Epub 2021 May 11. Neurosci Lett. 2021. PMID: 33989730
-
Recombinant adeno-associated virus as delivery vector for gene therapy--a review.Stem Cells Dev. 2004 Feb;13(1):133-45. doi: 10.1089/154732804773099335. Stem Cells Dev. 2004. PMID: 15068701 Review.
-
Expressing Transgenes That Exceed the Packaging Capacity of Adeno-Associated Virus Capsids.Hum Gene Ther Methods. 2016 Feb;27(1):1-12. doi: 10.1089/hgtb.2015.140. Hum Gene Ther Methods. 2016. PMID: 26757051 Free PMC article. Review.
Cited by
-
Persistent transgene expression in peripheral tissues one year post intravenous and intramuscular administration of AAV vectors containing the alphaherpesvirus latency-associated promoter 2.Front Virol. 2024;4:1379991. doi: 10.3389/fviro.2024.1379991. Epub 2024 Mar 28. Front Virol. 2024. PMID: 38665693 Free PMC article.
References
-
- Tuszynski MH. Translational Neuroscience. New York, NY: Springer; 2016. 584 p.
Publication types
MeSH terms
Grants and funding
- P40 OD010996/OD/NIH HHS/United States
- 1U01NS113868/U.S. Department of Health & Human Services | NIH | Center for Information Technology (Center for Information Technology, National Institutes of Health)
- P40OD010996/U.S. Department of Health & Human Services | NIH | Center for Information Technology (Center for Information Technology, National Institutes of Health)
- UL1 TR003017/TR/NCATS NIH HHS/United States
- U01 NS113868/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

