Symbiodiniaceae photophysiology and stress resilience is enhanced by microbial associations
- PMID: 38007500
- PMCID: PMC10676399
- DOI: 10.1038/s41598-023-48020-9
Symbiodiniaceae photophysiology and stress resilience is enhanced by microbial associations
Abstract
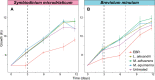
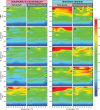
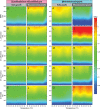
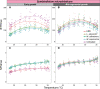
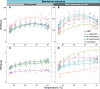
Symbiodiniaceae form associations with extra- and intracellular bacterial symbionts, both in culture and in symbiosis with corals. Bacterial associates can regulate Symbiodiniaceae fitness in terms of growth, calcification and photophysiology. However, the influence of these bacteria on interactive stressors, such as temperature and light, which are known to influence Symbiodiniaceae physiology, remains unclear. Here, we examined the photophysiological response of two Symbiodiniaceae species (Symbiodinium microadriaticum and Breviolum minutum) cultured under acute temperature and light stress with specific bacterial partners from their microbiome (Labrenzia (Roseibium) alexandrii, Marinobacter adhaerens or Muricauda aquimarina). Overall, bacterial presence positively impacted Symbiodiniaceae core photosynthetic health (photosystem II [PSII] quantum yield) and photoprotective capacity (non-photochemical quenching; NPQ) compared to cultures with all extracellular bacteria removed, although specific benefits were variable across Symbiodiniaceae genera and growth phase. Symbiodiniaceae co-cultured with M. aquimarina displayed an inverse NPQ response under high temperatures and light, and those with L. alexandrii demonstrated a lowered threshold for induction of NPQ, potentially through the provision of antioxidant compounds such as zeaxanthin (produced by Muricauda spp.) and dimethylsulfoniopropionate (DMSP; produced by this strain of L. alexandrii). Our co-culture approach empirically demonstrates the benefits bacteria can deliver to Symbiodiniaceae photochemical performance, providing evidence that bacterial associates can play important functional roles for Symbiodiniaceae.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Photophysiological response of Symbiodiniaceae single cells to temperature stress.ISME J. 2022 Aug;16(8):2060-2064. doi: 10.1038/s41396-022-01243-6. Epub 2022 Apr 26. ISME J. 2022. PMID: 35474114 Free PMC article.
-
Coral endosymbiont growth is enhanced by metabolic interactions with bacteria.Nat Commun. 2023 Oct 27;14(1):6864. doi: 10.1038/s41467-023-42663-y. Nat Commun. 2023. PMID: 37891154 Free PMC article.
-
Individual and combined effects of herbicide prometryn and nitrate enrichment at environmentally relevant concentrations on photosynthesis, oxidative stress, and endosymbiont community diversity of coral Acropora hyacinthus.Chemosphere. 2023 Oct;339:139729. doi: 10.1016/j.chemosphere.2023.139729. Epub 2023 Aug 3. Chemosphere. 2023. PMID: 37543226
-
Symbiodiniaceae-bacteria interactions: rethinking metabolite exchange in reef-building corals as multi-partner metabolic networks.Environ Microbiol. 2020 May;22(5):1675-1687. doi: 10.1111/1462-2920.14918. Epub 2020 Jan 23. Environ Microbiol. 2020. PMID: 31943674 Review.
-
Gene clusters for biosynthesis of mycosporine-like amino acids in dinoflagellate nuclear genomes: Possible recent horizontal gene transfer between species of Symbiodiniaceae (Dinophyceae).J Phycol. 2022 Feb;58(1):1-11. doi: 10.1111/jpy.13219. Epub 2021 Nov 26. J Phycol. 2022. PMID: 34699617 Free PMC article. Review.
Cited by
-
Chlamydiae as symbionts of photosynthetic dinoflagellates.ISME J. 2024 Jan 8;18(1):wrae139. doi: 10.1093/ismejo/wrae139. ISME J. 2024. PMID: 39046276 Free PMC article.
-
Photosynthesis and other factors affecting the establishment and maintenance of cnidarian-dinoflagellate symbiosis.Philos Trans R Soc Lond B Biol Sci. 2024 May 6;379(1901):20230079. doi: 10.1098/rstb.2023.0079. Epub 2024 Mar 18. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 38497261
-
Lipid Droplets in Endosymbiotic Symbiodiniaceae spp. Associated with Corals.Plants (Basel). 2024 Mar 25;13(7):949. doi: 10.3390/plants13070949. Plants (Basel). 2024. PMID: 38611478 Free PMC article. Review.
References
-
- Santos SR, Taylor DJ, Coffroth MA. Genetic comparisons of freshly isolated versus cultured symbiotic dinoflagellates: Implications for extrapolating to the intact symbiosis. J. Phycol. 2001;37:900–912. doi: 10.1046/j.1529-8817.2001.00194.x. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

