A hypothesis: Potential contributions of metals to the pathogenesis of pulmonary artery hypertension
- PMID: 38007143
- PMCID: PMC10872724
- DOI: 10.1016/j.lfs.2023.122289
A hypothesis: Potential contributions of metals to the pathogenesis of pulmonary artery hypertension
Abstract
Pulmonary artery hypertension (PAH) is characterized by vasoconstriction and vascular remodeling resulting in both increased pulmonary vascular resistance (PVR) and pulmonary artery pressure (PAP). The chronic and high-pressure stress experienced by endothelial cells can give rise to inflammation, oxidative stress, and infiltration by immune cells. However, there is no clearly defined mechanism for PAH and available treatment options only provide limited symptomatic relief. Due to the far-reaching effects of metal exposures, the interaction between metals and the pulmonary vasculature is of particular interest. This review will briefly introduce the pathophysiology of PAH and then focus on the potential roles of metals, including essential and non-essential metals in the pathogenic process in the pulmonary arteries and right heart, which may be linked to PAH. Based on available data from human studies of occupational or environmental metal exposure, including lead, antimony, iron, and copper, the hypothesis of metals contributing to the pathogenesis of PAH is proposed as potential risk factors and underlying mechanisms for PAH. We propose that metals may initiate or exacerbate the pathogenesis of PAH, by providing potential mechanism by which metals interact with hypoxia-inducible factor and tumor suppressor p53 to modulate their downstream cellular proliferation pathways. These need further investigation. Additionally, we present future research directions on roles of metals in PAH.
Keywords: Heavy metals; Mineral homeostasis; Non-essential metals; Pulmonary hypertension; Pulmonary hypoxia; Right ventricle dysfunction; Trace elements.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest NA.
Figures
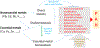

Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Guanylate cyclase stimulators for pulmonary hypertension.Cochrane Database Syst Rev. 2016 Aug 2;2016(8):CD011205. doi: 10.1002/14651858.CD011205.pub2. Cochrane Database Syst Rev. 2016. PMID: 27482837 Free PMC article. Review.
-
Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Unlocking data: Decision-maker perspectives on cross-sectoral data sharing and linkage as part of a whole-systems approach to public health policy and practice.Public Health Res (Southampt). 2024 Nov 20:1-30. doi: 10.3310/KYTW2173. Online ahead of print. Public Health Res (Southampt). 2024. PMID: 39582242
References
-
- Humbert M., et al., 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J, 2023. 61(1). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

