The Role of Cyanidin-3- O-glucoside in Modulating Oxaliplatin Resistance by Reversing Mesenchymal Phenotype in Colorectal Cancer
- PMID: 38004099
- PMCID: PMC10674439
- DOI: 10.3390/nu15224705
The Role of Cyanidin-3- O-glucoside in Modulating Oxaliplatin Resistance by Reversing Mesenchymal Phenotype in Colorectal Cancer
Abstract
Background: Epithelial-mesenchymal transition (EMT) plays an important role in the biological and biochemical processes of cells, and it is a critical process in the malignant transformation, and mobility of cancer. Additionally, EMT is one of the main mechanisms contributing to chemoresistance. Resistance to oxaliplatin (OXA) poses a momentous challenge in the chemotherapy of advanced colorectal cancer (CRC) patients, highlighting the need to reverse drug resistance and improve patient survival. In this study, we explored the response of cyanidin-3-O-glucoside (C3G), the most abundant anthocyanin in plants, on the mechanisms of drug resistance in cancer, with the purpose of overcoming acquired OXA resistance in CRC cell lines.
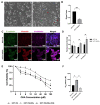
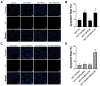
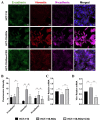
Methods: We generated an acquired OXA-resistant cell line, named HCT-116-ROx, by gradually exposing parental HCT-116 cells to increasing concentrations of OXA. To characterize the resistance, we performed cytotoxicity assays and shape factor analyses. The apoptotic rate of both resistant and parental cells was determined using Hoechst 33342/Propidium Iodide (PI) fluorescence staining. Migration capacity was evaluated using a wound-healing assay. The mesenchymal phenotype was assessed through qRT-PCR and immunofluorescence staining, employing E-cadherin, N-cadherin, and Vimentin markers.
Results: Resistance characterization announced decreased OXA sensitivity in resistant cells compared to parental cells. Moreover, the resistant cells exhibited a spindle cell morphology, indicative of the mesenchymal phenotype. Combined treatment of C3G and OXA resulted in an augmented apoptotic rate in the resistant cells. The migration capacity of resistant cells was higher than parental cells, while treatment with C3G decreased the migration rate of HCT-116-ROx cells. Analysis of EMT markers showed that HCT-116-ROx cells exhibited loss of the epithelial phenotype (E-cadherin) and gain of the mesenchymal phenotype (N-cadherin and Vimentin) compared to HCT-116 cells. However, treatment of resistant cells with C3G reversed the mesenchymal phenotype.
Conclusion: The morphological observations of cells acquiring oxaliplatin resistance indicated the loss of the epithelial phenotype and the acquisition of the mesenchymal phenotype. These findings suggest that EMT may contribute to acquired OXA resistance in CRC. Furthermore, C3G decreased the mobility of resistant cells, and reversed the EMT process, indicating its potential to overcome acquired OXA resistance.
Keywords: colorectal cancer; cyanidin-3-O-glucoside; epithelial-mesenchymal transition; oxaliplatin resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Cyanidin-3-glucoside induces mesenchymal to epithelial transition via activating Sirt1 expression in triple negative breast cancer cells.Biochimie. 2019 Jul;162:107-115. doi: 10.1016/j.biochi.2019.03.004. Epub 2019 Mar 12. Biochimie. 2019. PMID: 30876970
-
Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines.Clin Cancer Res. 2006 Jul 15;12(14 Pt 1):4147-53. doi: 10.1158/1078-0432.CCR-06-0038. Clin Cancer Res. 2006. PMID: 16857785
-
S100A6 mediated epithelial-mesenchymal transition affects chemosensitivity of colorectal cancer to oxaliplatin.Gene. 2024 Jul 1;914:148406. doi: 10.1016/j.gene.2024.148406. Epub 2024 Mar 22. Gene. 2024. PMID: 38521111
-
Unraveling the function of epithelial-mesenchymal transition (EMT) in colorectal cancer: Metastasis, therapy response, and revisiting molecular pathways.Biomed Pharmacother. 2023 Apr;160:114395. doi: 10.1016/j.biopha.2023.114395. Epub 2023 Feb 15. Biomed Pharmacother. 2023. PMID: 36804124 Review.
-
Role of Epithelial to Mesenchymal Transition in Colorectal Cancer.Int J Mol Sci. 2023 Oct 1;24(19):14815. doi: 10.3390/ijms241914815. Int J Mol Sci. 2023. PMID: 37834263 Free PMC article. Review.
References
-
- Dekker E., Tanis P.J., Vleugels J.L.A., Kasi P.M., Wallace M.B. Colorectal cancer. Lancet. 2019;394:1467–1480. - PubMed
-
- Brenner H., Kloor M., Pox C.P. Colorectal cancer. Lancet. 2014;383:1490–1502. - PubMed
-
- Buccafusca G., Proserpio I., Tralongo A.C., Giuliano S.R., Tralongo P. Early colorectal cancer: Diagnosis, treatment and survivorship care. Crit. Rev. Oncol. Hematol. 2019;136:20–30. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

