Role of CB1 Cannabinoid Receptors in Vascular Responses and Vascular Remodeling of the Aorta in Female Mice
- PMID: 38003619
- PMCID: PMC10671338
- DOI: 10.3390/ijms242216429
Role of CB1 Cannabinoid Receptors in Vascular Responses and Vascular Remodeling of the Aorta in Female Mice
Abstract
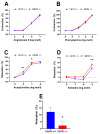
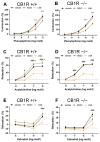
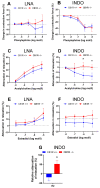
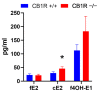
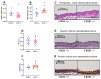
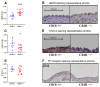
Both the endocannabinoid system (ECS) and estrogens have significant roles in cardiovascular control processes. Cannabinoid type 1 receptors (CB1Rs) mediate acute vasodilator and hypotensive effects, although their role in cardiovascular pathological conditions is still controversial. Estrogens exert cardiovascular protection in females. We aimed to study the impact of ECS on vascular functions. Experiments were performed on CB1R knockout (CB1R KO) and wild-type (WT) female mice. Plasma estrogen metabolite levels were determined. Abdominal aortas were isolated for myography and histology. Vascular effects of phenylephrine (Phe), angiotensin II, acetylcholine (Ach) and estradiol (E2) were obtained and repeated with inhibitors of nitric oxide synthase (NOS, Nω-nitro-L-arginine) and of cyclooxygenase (COX, indomethacin). Histological stainings (hematoxylin-eosin, resorcin-fuchsin) and immunostainings for endothelial NOS (eNOS), COX-2, estrogen receptors (ER-α, ER-β) were performed. Conjugated E2 levels were higher in CB1R KO compared to WT mice. Vasorelaxation responses to Ach and E2 were increased in CB1R KO mice, attenuated by NOS-inhibition. COX-inhibition decreased Phe-contractions, while it increased Ach-relaxation in the WT group but not in the CB1R KO. Effects of indomethacin on E2-relaxation in CB1R KO became opposite to that observed in WT. Histology revealed lower intima/media thickness and COX-2 density, higher eNOS and lower ER-β density in CB1R KO than in WT mice. CB1R KO female mice are characterized by increased vasorelaxation associated with increased utilization of endothelial NO and a decreased impact of constrictor prostanoids. Our results indicate that the absence or inhibition of CB1Rs may have beneficial vascular effects.
Keywords: cannabinoid type 1 receptor; endocannabinoid; endothelium; estrogen receptor; vascular remodeling.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
Investigating the Role of Cannabinoid Type 1 Receptors in Vascular Function and Remodeling in a Hypercholesterolemic Mouse Model with Low-Density Lipoprotein-Cannabinoid Type 1 Receptor Double Knockout Animals.Int J Mol Sci. 2024 Sep 2;25(17):9537. doi: 10.3390/ijms25179537. Int J Mol Sci. 2024. PMID: 39273484 Free PMC article.
-
Adaptive increases in expression and vasodilator activity of estrogen receptor subtypes in a blood vessel-specific pattern during pregnancy.Am J Physiol Heart Circ Physiol. 2015 Nov 15;309(10):H1679-96. doi: 10.1152/ajpheart.00532.2015. Epub 2015 Sep 25. Am J Physiol Heart Circ Physiol. 2015. PMID: 26408543 Free PMC article.
-
Subtype-specific estrogen receptor-mediated vasodilator activity in the cephalic, thoracic, and abdominal vasculature of female rat.J Cardiovasc Pharmacol. 2013 Jul;62(1):26-40. doi: 10.1097/FJC.0b013e31828bc88a. J Cardiovasc Pharmacol. 2013. PMID: 23429596 Free PMC article.
-
Increased expression of cyclooxygenase-2 mediates enhanced contraction to endothelin ETA receptor stimulation in endothelial nitric oxide synthase knockout mice.Circ Res. 2006 Jun 9;98(11):1439-45. doi: 10.1161/01.RES.0000224120.52792.10. Epub 2006 Apr 27. Circ Res. 2006. PMID: 16645140
-
Sensitivity of NOS-dependent vascular relaxation pathway to mineralocorticoid receptor blockade in caveolin-1-deficient mice.Am J Physiol Heart Circ Physiol. 2010 Jun;298(6):H1776-88. doi: 10.1152/ajpheart.00661.2009. Epub 2010 Apr 2. Am J Physiol Heart Circ Physiol. 2010. PMID: 20363891 Free PMC article.
Cited by
-
Investigating the Role of Cannabinoid Type 1 Receptors in Vascular Function and Remodeling in a Hypercholesterolemic Mouse Model with Low-Density Lipoprotein-Cannabinoid Type 1 Receptor Double Knockout Animals.Int J Mol Sci. 2024 Sep 2;25(17):9537. doi: 10.3390/ijms25179537. Int J Mol Sci. 2024. PMID: 39273484 Free PMC article.
References
-
- Szekeres M., Nádasy G.L., Turu G., Soltész-Katona E., Benyó Z., Offermanns S., Ruisanchez É., Szabó E., Takáts Z., Bátkai S., et al. Endocannabinoid-mediated modulation of Gq/11 protein-coupled receptor signaling-induced vasoconstriction and hypertension. Mol. Cell. Endocrinol. 2015;403:46–56. doi: 10.1016/j.mce.2015.01.012. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

