Identification of CDK1, PBK, and CHEK1 as an Oncogenic Signature in Glioblastoma: A Bioinformatics Approach to Repurpose Dapagliflozin as a Therapeutic Agent
- PMID: 38003585
- PMCID: PMC10671581
- DOI: 10.3390/ijms242216396
Identification of CDK1, PBK, and CHEK1 as an Oncogenic Signature in Glioblastoma: A Bioinformatics Approach to Repurpose Dapagliflozin as a Therapeutic Agent
Abstract
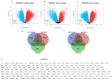
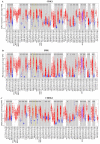
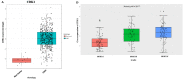
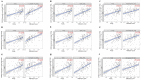
Glioblastoma multiforme (GBM) is the most aggressive and lethal primary brain tumor whose median survival is less than 15 months. The current treatment regimen comprising surgical resectioning, chemotherapy with Temozolomide (TMZ), and adjuvant radiotherapy does not achieve total patient cure. Stem cells' presence and GBM tumor heterogeneity increase their resistance to TMZ, hence the poor overall survival of patients. A dysregulated cell cycle in glioblastoma enhances the rapid progression of GBM by evading senescence or apoptosis through an over-expression of cyclin-dependent kinases and other protein kinases that are the cell cycle's main regulatory proteins. Herein, we identified and validated the biomarker and predictive properties of a chemoradio-resistant oncogenic signature in GBM comprising CDK1, PBK, and CHEK1 through our comprehensive in silico analysis. We found that CDK1/PBK/CHEK1 overexpression drives the cell cycle, subsequently promoting GBM tumor progression. In addition, our Kaplan-Meier survival estimates validated the poor patient survival associated with an overexpression of these genes in GBM. We used in silico molecular docking to analyze and validate our objective to repurpose Dapagliflozin against CDK1/PBK/CHEK1. Our results showed that Dapagliflozin forms putative conventional hydrogen bonds with CDK1, PBK, and CHEK1 and arrests the cell cycle with the lowest energies as Abemaciclib.
Keywords: Abemaciclib; Dapagliflozin; PDZ binding kinase; Temozolomide; cell cycle; checkpoint kinase 1; cyclin-dependent kinase 1; drug repurposing; glioblastoma multiforme; molecular docking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Inhibition of cyclin E1 overcomes temozolomide resistance in glioblastoma by Mcl-1 degradation.Mol Carcinog. 2019 Aug;58(8):1502-1511. doi: 10.1002/mc.23034. Epub 2019 May 2. Mol Carcinog. 2019. PMID: 31045274
-
miR-140 targeting CTSB signaling suppresses the mesenchymal transition and enhances temozolomide cytotoxicity in glioblastoma multiforme.Pharmacol Res. 2019 Sep;147:104390. doi: 10.1016/j.phrs.2019.104390. Epub 2019 Aug 6. Pharmacol Res. 2019. PMID: 31398406
-
Exploring the Functional Roles of Telomere Maintenance 2 in the Tumorigenesis of Glioblastoma Multiforme and Drug Responsiveness to Temozolomide.Int J Mol Sci. 2023 May 25;24(11):9256. doi: 10.3390/ijms24119256. Int J Mol Sci. 2023. PMID: 37298208 Free PMC article.
-
Dysregulated lipid metabolism in TMZ-resistant glioblastoma: pathways, proteins, metabolites and therapeutic opportunities.Lipids Health Dis. 2023 Aug 3;22(1):114. doi: 10.1186/s12944-023-01881-5. Lipids Health Dis. 2023. PMID: 37537607 Free PMC article. Review.
-
Involvement of Intracellular Cholesterol in Temozolomide-Induced Glioblastoma Cell Death.Neurol Med Chir (Tokyo). 2018 Jul 15;58(7):296-302. doi: 10.2176/nmc.ra.2018-0040. Epub 2018 Jun 13. Neurol Med Chir (Tokyo). 2018. PMID: 29899179 Free PMC article. Review.
Cited by
-
Unveiling the anticancer effects of SGLT-2i: mechanisms and therapeutic potential.Front Pharmacol. 2024 Mar 26;15:1369352. doi: 10.3389/fphar.2024.1369352. eCollection 2024. Front Pharmacol. 2024. PMID: 38595915 Free PMC article. Review.
-
Comprehensive analysis of bulk and single-cell RNA sequencing data reveals Schlafen-5 (SLFN5) as a novel prognosis and immunity.Int J Med Sci. 2024 Sep 9;21(12):2348-2364. doi: 10.7150/ijms.97975. eCollection 2024. Int J Med Sci. 2024. PMID: 39310264 Free PMC article.
-
Discovery of key molecular signatures for diagnosis and therapies of glioblastoma by combining supervised and unsupervised learning approaches.Sci Rep. 2024 Nov 11;14(1):27545. doi: 10.1038/s41598-024-79391-2. Sci Rep. 2024. PMID: 39528802 Free PMC article.
References
-
- Joel M., Mughal A.A., Grieg Z., Murrell W., Palmero S., Mikkelsen B., Fjerdingstad H.B., Sandberg C.J., Behnan J., Glover J.C., et al. Targeting PBK/TOPK decreases growth and survival of glioma initiating cells in vitro and attenuates tumor growth in vivo. Mol. Cancer. 2015;14:121. doi: 10.1186/s12943-015-0398-x. - DOI - PMC - PubMed
-
- Signore M., Pelacchi F., Di Martino S., Runci D., Biffoni M., Giannetti S., Morgante L., De Majo M., Petricoin E., Stancato L. Combined PDK1 and CHK1 inhibition is required to kill glioblastoma stem-like cells in vitro and in vivo. Cell Death Dis. 2014;5:e1223. doi: 10.1038/cddis.2014.188. - DOI - PMC - PubMed
-
- Ostrom Q.T., Gittleman H., Farah P., Ondracek A., Chen Y., Wolinsky Y., Stroup N.E., Kruchko C., Barnholtz-Sloan J.S. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro-Oncology. 2013;15((Suppl. S2)):ii1–ii56. doi: 10.1093/neuonc/not151. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 112-2314-B-038 -019-/National Science and Technology Council
- TMU111-AE2-I14-3/Taipei Medical University
- 111TMU-WFH-10/Taipei Medical University and Wan-Fang Hospital Research
- NSTC112-2314-B-038-006/The National Science and Technology Council, Taiwan
- SKH-TMU-112-02/Shin Kong Wu Ho-Su Memorial Hospital
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

