Diastereoselective Synthesis of Dispiro[Imidazothiazolotriazine-Pyrrolidin-Oxindoles] and Their Isomerization Pathways in Basic Medium
- PMID: 38003560
- PMCID: PMC10671214
- DOI: 10.3390/ijms242216359
Diastereoselective Synthesis of Dispiro[Imidazothiazolotriazine-Pyrrolidin-Oxindoles] and Their Isomerization Pathways in Basic Medium
Abstract
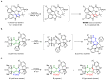
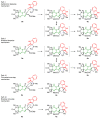
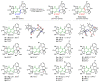
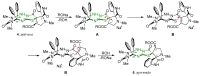
Highly diastereoselective methods for the synthesis of two series of regioisomeric polynuclear dispyroheterocyclic compounds with five or six chiral centers, comprising moieties of pyrrolidinyloxindole and imidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine of linear structure or imidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine of angular structure, have been developed on the basis of a [3+2] cycloaddition of azomethine ylides to functionalized imidazothiazolotriazines. Depending on the structure of the ethylenic component, cycloaddition proceeds as an anti-exo process for linear derivatives, while cycloaddition to angular ones resulted in a syn-endo diastereomer. Novel pathways of isomerization for the synthesized anti-exo products upon treatment with sodium alkoxides have been found, which resulted in two more series of diastereomeric dispiro[imidazothiazolotriazine-pyrrolidin-oxindoles] inaccessible with the direct cycloaddition reaction. For the first series, the inversion of the configuration of one stereocenter, i.e., C-4' atom of the pyrrolidine cycle, (epimerization) was established. For the second one, configuration of the obtained diastereomer formally corresponded to the syn-endo approach of the azomethine ylide in the case of cycloaddition to the ethylenic component.
Keywords: [3+2] cycloaddition; azomethine ylides; dispirooxindoles; epimerization; imidazothiazolotriazines; isomerization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
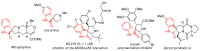

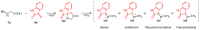
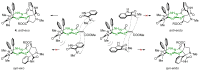
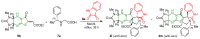
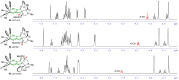
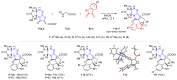
Similar articles
-
Synthesis, Structure and Stereochemistry of Dispirocompounds Based on Imidazothiazolotriazine and Pyrrolidineoxindole.Int J Mol Sci. 2022 Nov 10;23(22):13820. doi: 10.3390/ijms232213820. Int J Mol Sci. 2022. PMID: 36430300 Free PMC article.
-
Diastereodivergent synthesis of dispiroheterocyclic structures comprising pyrrolidinyloxindole and imidazothiazolotriazine moieties.Org Biomol Chem. 2020 Sep 21;18(35):6905-6911. doi: 10.1039/d0ob01628d. Epub 2020 Aug 28. Org Biomol Chem. 2020. PMID: 32856678
-
An effective one-pot access to polynuclear dispiroheterocyclic structures comprising pyrrolidinyloxindole and imidazothiazolotriazine moieties via a 1,3-dipolar cycloaddition strategy.Beilstein J Org Chem. 2016 Oct 24;12:2240-2249. doi: 10.3762/bjoc.12.216. eCollection 2016. Beilstein J Org Chem. 2016. PMID: 28144290 Free PMC article.
-
1,3-Dipolar cycloaddition reactions of isatin-derived azomethine ylides for the synthesis of spirooxindole and indole-derived scaffolds: recent developments.Mol Divers. 2023 Oct;27(5):2365-2397. doi: 10.1007/s11030-022-10510-9. Epub 2022 Aug 4. Mol Divers. 2023. PMID: 35925529 Review.
-
1,3-Dipolar Cycloaddition Reactions of Azomethine Ylides with Carbonyl Dipolarophiles Yielding Oxazolidine Derivatives.Molecules. 2016 Jul 23;21(8):935. doi: 10.3390/molecules21080935. Molecules. 2016. PMID: 27455230 Free PMC article. Review.
References
-
- Jossang A., Jossang P., Hadi H.A., Sevenet T., Bodo B. Horsfiline, an oxindole alkaloid from Horsfieldia superba. J. Org. Chem. 1991;56:6527–6530. doi: 10.1021/jo00023a016. - DOI
-
- Stuppner H., Sturm S., Konwalinka G. HPLC analysis of the main oxindole alkaloids from Uncaria tomentosa. Chromatographia. 1992;34:597–600. doi: 10.1007/BF02269869. - DOI
-
- Ivanenkov Y.A., Kukushkin M.E., Beloglazkina A.A., Shafikov R.R., Barashkin A.A., Ayginin A.A., Serebryakova M.S., Majouga A.G., Skvortsov D.A., Tafeenko V.A., et al. Synthesis and Biological Evaluation of Novel Dispiro-Indolinones with Anticancer Activity. Molecules. 2023;28:1325. doi: 10.3390/molecules28031325. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

