The Effects of Deregulated Ribosomal Biogenesis in Cancer
- PMID: 38002277
- PMCID: PMC10669593
- DOI: 10.3390/biom13111593
The Effects of Deregulated Ribosomal Biogenesis in Cancer
Abstract
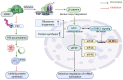
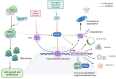
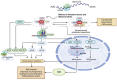
Ribosomes are macromolecular ribonucleoprotein complexes assembled from RNA and proteins. Functional ribosomes arise from the nucleolus, require ribosomal RNA processing and the coordinated assembly of ribosomal proteins (RPs), and are frequently hyperactivated to support the requirement for protein synthesis during the self-biosynthetic and metabolic activities of cancer cells. Studies have provided relevant information on targeted anticancer molecules involved in ribosome biogenesis (RiBi), as increased RiBi is characteristic of many types of cancer. The association between unlimited cell proliferation and alterations in specific steps of RiBi has been highlighted as a possible critical driver of tumorigenesis and metastasis. Thus, alterations in numerous regulators and actors involved in RiBi, particularly in cancer, significantly affect the rate and quality of protein synthesis and, ultimately, the transcriptome to generate the associated proteome. Alterations in RiBi in cancer cells activate nucleolar stress response-related pathways that play important roles in cancer-targeted interventions and immunotherapies. In this review, we focus on the association between alterations in RiBi and cancer. Emphasis is placed on RiBi deregulation and its secondary consequences, including changes in protein synthesis, loss of RPs, adaptive transcription and translation, nucleolar stress regulation, metabolic changes, and the impaired ribosome biogenesis checkpoint.
Keywords: cell signaling pathways; metastasis; nucleolar stress regulation; ribosomal biogenesis; ribosomes; tumorigenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Nucleolar stress: From development to cancer.Semin Cell Dev Biol. 2023 Feb 28;136:64-74. doi: 10.1016/j.semcdb.2022.04.001. Epub 2022 Apr 8. Semin Cell Dev Biol. 2023. PMID: 35410715 Free PMC article. Review.
-
p53 at the crossroad of DNA replication and ribosome biogenesis stress pathways.Cell Death Differ. 2022 May;29(5):972-982. doi: 10.1038/s41418-022-00999-w. Epub 2022 Apr 20. Cell Death Differ. 2022. PMID: 35444234 Free PMC article. Review.
-
Additional principles that govern the release of pre-ribosomes from the nucleolus into the nucleoplasm in yeast.Nucleic Acids Res. 2023 Nov 10;51(20):10867-10883. doi: 10.1093/nar/gkac430. Nucleic Acids Res. 2023. PMID: 35736211 Free PMC article.
-
Ribosome Biogenesis: A Central Player in Cancer Metastasis and Therapeutic Resistance.Cancer Res. 2022 Jul 5;82(13):2344-2353. doi: 10.1158/0008-5472.CAN-21-4087. Cancer Res. 2022. PMID: 35303060 Free PMC article. Review.
-
The roles of RRP15 in nucleolar formation, ribosome biogenesis and checkpoint control in human cells.Oncotarget. 2017 Feb 21;8(8):13240-13252. doi: 10.18632/oncotarget.14658. Oncotarget. 2017. PMID: 28099941 Free PMC article.
Cited by
-
Ribosomal protein S3A (RPS3A), as a transcription regulator of colony-stimulating factor 1 (CSF1), promotes glioma progression through regulating the recruitment and autophagy-mediated M2 polarization of tumor-associated macrophages.Naunyn Schmiedebergs Arch Pharmacol. 2024 Nov 19. doi: 10.1007/s00210-024-03601-x. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 39560749
-
Multiple myeloma: clinical characteristics, current therapies and emerging innovative treatments targeting ribosome biogenesis dynamics.Clin Exp Metastasis. 2024 Dec;41(6):829-842. doi: 10.1007/s10585-024-10305-2. Epub 2024 Aug 20. Clin Exp Metastasis. 2024. PMID: 39162964 Free PMC article. Review.
-
Ribosome biogenesis and ribosomal proteins in cancer stem cells: a new therapeutic prospect.Mol Biol Rep. 2024 Sep 26;51(1):1016. doi: 10.1007/s11033-024-09963-y. Mol Biol Rep. 2024. PMID: 39325314 Review.
References
-
- Zolotenkova E.A., Gopanenko A.V., Tupikin A.E., Kabilov M.R., Malygin A.A. Mutation at the Site of Hydroxylation in the Ribosomal Protein uL15 (RPL27a) Causes Specific Changes in the Repertoire of mRNAs Translated in Mammalian Cells. Int. J. Mol. Sci. 2023;24:6173. doi: 10.3390/ijms24076173. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

