Prostaglandin E2 Boosts the Hyaluronan-Mediated Increase in Inflammatory Response to Lipopolysaccharide by Enhancing Lyve1 Expression
- PMID: 37998039
- PMCID: PMC10669677
- DOI: 10.3390/biology12111441
Prostaglandin E2 Boosts the Hyaluronan-Mediated Increase in Inflammatory Response to Lipopolysaccharide by Enhancing Lyve1 Expression
Abstract
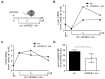
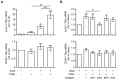
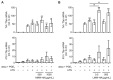
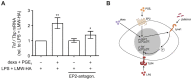
Macrophages are a highly versatile and heterogenic group of immune cells, known for their involvement in inflammatory reactions. However, our knowledge about distinct subpopulations of macrophages and their specific contribution to the resolution of inflammation remains incomplete. We have previously shown, in an in vivo peritonitis model, that inhibition of the synthesis of the pro-inflammatory lipid mediator prostaglandin E2 (PGE2) attenuates efficient resolution of inflammation. PGE2 levels during later stages of the inflammatory process further correlate with expression of the hyaluronan (HA) receptor Lyve1 in peritoneal macrophages. In the present study, we therefore aimed to understand if PGE2 might contribute to the regulation of Lyve1 and how this might impact inflammatory responses. In line with our in vivo findings, PGE2 synergized with dexamethasone to enhance Lyve1 expression in bone marrow-derived macrophages, while expression of the predominant hyaluronan receptor CD44 remained unaltered. PGE2-mediated Lyve1 upregulation was strictly dependent on PGE2 receptor EP2 signaling. While PGE2/dexamethasone-treated macrophages, despite their enhanced Lyve1 expression, did not show inflammatory responses upon stimulation with low (LMW) or high-molecular-weight hyaluronan (HMW)-HA, they were sensitized towards LMW-HA-dependent augmentation of lipopolysaccharide (LPS)-induced inflammatory responses. Thus, Lyve1-expressing macrophages emerged as a subpopulation of macrophages integrating inflammatory stimuli with extracellular matrix-derived signals.
Keywords: hyaluronic acid; inflammation; macrophage; peritonitis; resolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Low molecular weight hyaluronan activates cytosolic phospholipase A2α and eicosanoid production in monocytes and macrophages.J Biol Chem. 2014 Feb 14;289(7):4470-88. doi: 10.1074/jbc.M113.515106. Epub 2013 Dec 23. J Biol Chem. 2014. PMID: 24366870 Free PMC article. Clinical Trial.
-
Induction of IL-12 and chemokines by hyaluronan requires adhesion-dependent priming of resident but not elicited macrophages.J Immunol. 1997 Sep 1;159(5):2492-500. J Immunol. 1997. PMID: 9278343
-
Low molecular weight hyaluronan mediated CD44 dependent induction of IL-6 and chemokines in human dermal fibroblasts potentiates innate immune response.Cytokine. 2014 Dec;70(2):97-103. doi: 10.1016/j.cyto.2014.07.006. Epub 2014 Aug 10. Cytokine. 2014. PMID: 25126764
-
Prostaglandin E2 and Its Receptor EP2 Modulate Macrophage Activation and Fusion in Vitro.ACS Biomater Sci Eng. 2020 May 11;6(5):2668-2681. doi: 10.1021/acsbiomaterials.9b01180. Epub 2020 Feb 17. ACS Biomater Sci Eng. 2020. PMID: 33463295
-
Lung Hyaluronasome: Involvement of Low Molecular Weight Ha (Lmw-Ha) in Innate Immunity.Biomolecules. 2022 Apr 30;12(5):658. doi: 10.3390/biom12050658. Biomolecules. 2022. PMID: 35625586 Free PMC article. Review.
Cited by
-
Developmental and functional roles of androgen and interactive signals for external genitalia and erectile tissues.Reprod Med Biol. 2024 Oct 6;23(1):e12611. doi: 10.1002/rmb2.12611. eCollection 2024 Jan-Dec. Reprod Med Biol. 2024. PMID: 39372370 Free PMC article. Review.
References
-
- Sindrilaru A., Peters T., Wieschalka S., Baican C., Baican A., Peter H., Hainzl A., Schatz S., Qi Y., Schlecht A., et al. An Unrestrained Proinflammatory M1 Macrophage Population Induced by Iron Impairs Wound Healing in Humans and Mice. J. Clin. Investig. 2011;121:985–997. doi: 10.1172/JCI44490. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

