Characterisation and prion transmission study in mice with genetic reduction of sporadic Creutzfeldt-Jakob disease risk gene Stx6
- PMID: 37996040
- PMCID: PMC7615600
- DOI: 10.1016/j.nbd.2023.106363
Characterisation and prion transmission study in mice with genetic reduction of sporadic Creutzfeldt-Jakob disease risk gene Stx6
Abstract
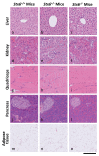
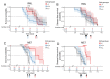
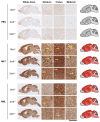
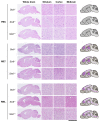
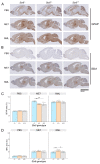
Sporadic Creutzfeldt-Jakob disease (sCJD), the most common human prion disease, is thought to occur when the cellular prion protein (PrPC) spontaneously misfolds and assembles into prion fibrils, culminating in fatal neurodegeneration. In a genome-wide association study of sCJD, we recently identified risk variants in and around the gene STX6, with evidence to suggest a causal increase of STX6 expression in disease-relevant brain regions. STX6 encodes syntaxin-6, a SNARE protein primarily involved in early endosome to trans-Golgi network retrograde transport. Here we developed and characterised a mouse model with genetic depletion of Stx6 and investigated a causal role of Stx6 expression in mouse prion disease through a classical prion transmission study, assessing the impact of homozygous and heterozygous syntaxin-6 knockout on disease incubation periods and prion-related neuropathology. Following inoculation with RML prions, incubation periods in Stx6-/- and Stx6+/- mice differed by 12 days relative to wildtype. Similarly, in Stx6-/- mice, disease incubation periods following inoculation with ME7 prions also differed by 12 days. Histopathological analysis revealed a modest increase in astrogliosis in ME7-inoculated Stx6-/- animals and a variable effect of Stx6 expression on microglia activation, however no differences in neuronal loss, spongiform change or PrP deposition were observed at endpoint. Importantly, Stx6-/- mice are viable and fertile with no gross impairments on a range of neurological, biochemical, histological and skeletal structure tests. Our results provide some support for a pathological role of Stx6 expression in prion disease, which warrants further investigation in the context of prion disease but also other neurodegenerative diseases considering syntaxin-6 appears to have pleiotropic risk effects in progressive supranuclear palsy and Alzheimer's disease.
Keywords: Creutzfeldt-Jakob disease; Incubation period; Prion disease; SNARE; Syntaxin-6.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest J.C. is a director and shareholder of D-Gen Limited, an academic spin-out company in the field of prion diagnosis, decontamination and therapeutics. There are no other competing interests.
Figures

Similar articles
-
Genetic risk factors for Creutzfeldt-Jakob disease.Neurobiol Dis. 2020 Aug;142:104973. doi: 10.1016/j.nbd.2020.104973. Epub 2020 Jun 18. Neurobiol Dis. 2020. PMID: 32565065 Review.
-
Absence of spontaneous disease and comparative prion susceptibility of transgenic mice expressing mutant human prion proteins.J Gen Virol. 2009 Mar;90(Pt 3):546-558. doi: 10.1099/vir.0.007930-0. J Gen Virol. 2009. PMID: 19218199 Free PMC article.
-
Identification of novel risk loci and causal insights for sporadic Creutzfeldt-Jakob disease: a genome-wide association study.Lancet Neurol. 2020 Oct;19(10):840-848. doi: 10.1016/S1474-4422(20)30273-8. Epub 2020 Sep 16. Lancet Neurol. 2020. PMID: 32949544 Free PMC article.
-
Prion Strain Characterization of a Novel Subtype of Creutzfeldt-Jakob Disease.J Virol. 2017 May 12;91(11):e02390-16. doi: 10.1128/JVI.02390-16. Print 2017 Jun 1. J Virol. 2017. PMID: 28298604 Free PMC article.
-
Recent advances in the histo-molecular pathology of human prion disease.Brain Pathol. 2019 Mar;29(2):278-300. doi: 10.1111/bpa.12695. Epub 2019 Jan 22. Brain Pathol. 2019. PMID: 30588685 Free PMC article. Review.
References
-
- Bock JB, Lin RC, Scheller RH. A new syntaxin family member implicated in targeting of intracellular transport vesicles. J Biol Chem. 1996;271(30):17961–17965. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

