Integrated analysis of Mendelian Randomization and Bayesian colocalization reveals bidirectional causal association between inflammatory bowel disease and psoriasis
- PMID: 37988718
- PMCID: PMC10836255
- DOI: 10.1080/07853890.2023.2281658
Integrated analysis of Mendelian Randomization and Bayesian colocalization reveals bidirectional causal association between inflammatory bowel disease and psoriasis
Abstract
Background: Observational studies have suggested an association between inflammatory bowel disease [IBD] and psoriasis. However, the detailed genetic basis, causality, and direction of this association remain unclear.
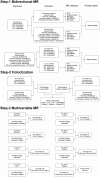
Methods: Bidirectional two-sample Mendelian Randomization [MR] analysis was conducted using summary statistics from published genome-wide association studies. Bayesian Colocalization and multivariable MR [MVMR] analyses were performed to identify candidate variants and risk genes involved in the shared genetic basis between IBD, psoriasis, and their subtypes.
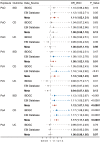
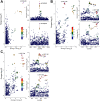
Results: Genetically predicted IBD and Crohn's disease [CD] were associated with an increased risk of psoriasis, psoriasis vulgaris [PsV], and psoriatic arthritis [PsA] (IBD on psoriasis: pooled odds ratio [OR] 1.09, 95% confidence interval [CI] 1.04-1.14, p = .0001; CD on psoriasis: pooled OR 1.10, 95% CI 1.06-1.15, p < .0001) and vice versa (psoriasis on IBD: pooled OR 1.11, 95%CI 1.02-1.21), whereas CD only exhibited a unidirectional association with psoriasis. Colocalization analysis revealed eight candidate genetic variants and risk genes (including LINC00824, CDKAL1, IL10, IL23R, DNAJC27, LPP, RUNX3, and RGS14) associated with a shared genetic basis. Among these, IL23R, DNAJC27, LPP, and RGS14 were further validated by MVMR analysis.
Conclusion: Our findings indicated bidirectional causal associations between IBD and psoriasis (including PsV and PsA), which were attributed primarily to CD rather than Ulcerative colitis [UC]. Furthermore, we identified several candidate variants and risk genes involved in the shared genetic basis of IBD and psoriasis. Acquiring a better understanding of the shared genetic architecture underlying IBD and psoriasis would help improve clinical strategies.
Keywords: Bayesian colocalization; Mendelian randomization; inflammatory bowel disease; psoriasis.
Conflict of interest statement
The authors declare that they have no competing interests.
We are currently conducting a study on bidirectional and have encountered some confusion. I want to figure out whether bidirectional MR analysis will make extra alpha errors and lead to overestimation of causal effects? In other words, do reverse causal effects affect the estimation of forward causal effects. If so, can we use a Bonferonni-corrected threshold to mitigate this effect. I have tried my best to searched in recently published articles about bidirectional MR analysis. However, there are no relevant descriptions about the disadvantages of bidirectional MR analysis. We would be grateful if you provide some professional insights.
I sincerely value and appreciate your contributions to the development of the MR field and look forward to your reply!
Best regards,
Siyuan Xie & Delong Chen
Figures


Similar articles
-
Causal Association Between Inflammatory Bowel Disease and Psoriasis: A Two-Sample Bidirectional Mendelian Randomization Study.Front Immunol. 2022 Jun 10;13:916645. doi: 10.3389/fimmu.2022.916645. eCollection 2022. Front Immunol. 2022. PMID: 35757704 Free PMC article.
-
Causality of inflammatory bowel disease and seborrheic keratosis: A bidirectional two-sample Mendelian randomization study.Skin Res Technol. 2024 Aug;30(8):e13876. doi: 10.1111/srt.13876. Skin Res Technol. 2024. PMID: 39081143 Free PMC article.
-
Inflammatory bowel disease and allergic diseases: A Mendelian randomization study.Pediatr Allergy Immunol. 2024 May;35(5):e14147. doi: 10.1111/pai.14147. Pediatr Allergy Immunol. 2024. PMID: 38773751
-
New IBD genetics: common pathways with other diseases.Gut. 2011 Dec;60(12):1739-53. doi: 10.1136/gut.2009.199679. Epub 2011 Feb 7. Gut. 2011. PMID: 21300624 Review.
-
Combining evidence from Mendelian randomization and colocalization: Review and comparison of approaches.Am J Hum Genet. 2022 May 5;109(5):767-782. doi: 10.1016/j.ajhg.2022.04.001. Epub 2022 Apr 21. Am J Hum Genet. 2022. PMID: 35452592 Free PMC article. Review.
Cited by
-
Unraveling the causal link: fatty acids and inflammatory bowel disease.Front Immunol. 2024 Jul 25;15:1405790. doi: 10.3389/fimmu.2024.1405790. eCollection 2024. Front Immunol. 2024. PMID: 39119343 Free PMC article.
-
Mendelian randomization analysis of psoriasis and psoriatic arthritis associated with risks of ulcerative colitis.Skin Res Technol. 2024 Jul;30(7):e13795. doi: 10.1111/srt.13795. Skin Res Technol. 2024. PMID: 38995229 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
