This is a preprint.
A Whole-Food, Plant-Based Randomized Controlled Trial in Metastatic Breast Cancer: Weight, Cardiometabolic, and Hormonal Outcome
- PMID: 37986940
- PMCID: PMC10659540
- DOI: 10.21203/rs.3.rs-3425125/v1
A Whole-Food, Plant-Based Randomized Controlled Trial in Metastatic Breast Cancer: Weight, Cardiometabolic, and Hormonal Outcome
Update in
-
A whole-food, plant-based randomized controlled trial in metastatic breast cancer: weight, cardiometabolic, and hormonal outcomes.Breast Cancer Res Treat. 2024 Jun;205(2):257-266. doi: 10.1007/s10549-024-07266-1. Epub 2024 Mar 6. Breast Cancer Res Treat. 2024. PMID: 38446316 Free PMC article. Clinical Trial.
Abstract
Purpose: Breast cancer treatment is associated with weight gain, and obesity and its related cardiometabolic and hormonal risk factors have been associated with poorer outcomes. Dietary intervention may address these risk factors, but limited research has been done in the setting of metastatic breast cancer requiring systemic therapy.
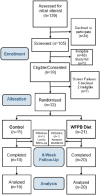
Methods: Women with metastatic breast cancer on stable treatment were randomized 2:1 to an 8-week intervention (n = 21) or control (n = 11). The intervention included weekly assessment visits and an ad libitum whole food, plant-based (WFPB) diet with provided meals. Cardiometabolic, hormonal, and cancer markers were assessed at baseline, 4 weeks, and 8 weeks.
Results: Within the intervention group, mean weight decreased by 6.6% (p < 0.01) after 8 weeks. Fasting insulin decreased from 16.8 uIU/L to 11.2 uIU/L (p < 0.01), concurrent with significantly reduced insulin resistance. Total cholesterol decreased from 193.6 mg/dL to 159 mg/dL (p < 0.01) and low-density lipoprotein (LDL) cholesterol decreased from 104.6 mg/dL to 82.2 mg/dL (p < 0.01). Total testosterone was unchanged, but free testosterone trended lower within the intervention group (p = 0.08) as sex hormone binding globulin increased from 74.3 nmol/L to 98.2 nmol/L (p < 0.01). There were no significant differences in cancer progression markers at week 8, although mean CA 15 - 3, CA 27.29, and CEA were lower in the intervention group (p = 0.53, p = 0.23, and p = 0.54, respectively) compared to control, when adjusted for baseline.
Conclusion: WFPB dietary changes during treatment for metastatic breast cancer are well tolerated and significantly improve weight and cardiometabolic and hormonal parameters. Longer studies are warranted to assess the durability of changes.
Trial registration: First registered at Clinicaltrials.gov (NCT03045289) on February 7, 2017.
Keywords: Breast cancer; Diet; Nutrition; Obesity; Plant-based diet; Vegan diet.
Conflict of interest statement
Competing Interests: TMC: Royalties from general interest books about plant-based nutrition (Benbella Books, Penguin Random House) and income from a lifestyle medicine practice, Thomas M. Campbell, MD PLLC; EKC: Conflicts of spouse (TMC); AH: MJH Healthcare Holdings (OncLive), Mediflix (Skipta/Informa); RGM: Consultant for Fujirebio Diagnostics. Research funding from Angle plc. The rest of the authors declare no competing interests.
Figures

Similar articles
-
A whole-food, plant-based randomized controlled trial in metastatic breast cancer: weight, cardiometabolic, and hormonal outcomes.Breast Cancer Res Treat. 2024 Jun;205(2):257-266. doi: 10.1007/s10549-024-07266-1. Epub 2024 Mar 6. Breast Cancer Res Treat. 2024. PMID: 38446316 Free PMC article. Clinical Trial.
-
A whole food, plant-based randomized controlled trial in metastatic breast cancer: feasibility, nutrient, and patient-reported outcomes.Breast Cancer Res Treat. 2024 Jul;206(2):273-283. doi: 10.1007/s10549-024-07284-z. Epub 2024 Mar 30. Breast Cancer Res Treat. 2024. PMID: 38553649 Free PMC article. Clinical Trial.
-
A Whole Food, Plant-Based Randomized Controlled Trial in Metastatic Breast Cancer: Feasibility, Nutrient, and Patient-Reported Outcomes.Res Sq [Preprint]. 2023 Nov 21:rs.3.rs-3606685. doi: 10.21203/rs.3.rs-3606685/v1. Res Sq. 2023. Update in: Breast Cancer Res Treat. 2024 Jun;205(2):257-266. doi: 10.1007/s10549-024-07266-1 PMID: 38045318 Free PMC article. Updated. Preprint.
-
Lipid Screening in Childhood for Detection of Multifactorial Dyslipidemia: A Systematic Evidence Review for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2016 Aug. Report No.: 14-05204-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016 Aug. Report No.: 14-05204-EF-1. PMID: 27559550 Free Books & Documents. Review.
-
Behavioral Counseling to Promote a Healthful Diet and Physical Activity for Cardiovascular Disease Prevention in Adults Without Known Cardiovascular Disease Risk Factors: Updated Systematic Review for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2017 Jul. Report No.: 15-05222-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2017 Jul. Report No.: 15-05222-EF-1. PMID: 29364620 Free Books & Documents. Review.
References
-
- Ligibel JA, Huebner L, Rugo HS, Burstein HJ, Toppmeyer DL, Anders CK, Ma C, Barry WT, Suman V, Carey LA et al.: Physical Activity, Weight, and Outcomes in Patients Receiving Chemotherapy for Metastatic Breast Cancer (C40502/Alliance). JNCI Cancer Spectr 2021, 5(3) DOI: 10.1093/jncics/pkab025. - DOI - PMC - PubMed
Publication types
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

