AGEs and RAGE: metabolic and molecular signatures of the glycation-inflammation axis in malignant or metastatic cancers
- PMID: 37970208
- PMCID: PMC10645465
- DOI: 10.37349/etat.2023.00170
AGEs and RAGE: metabolic and molecular signatures of the glycation-inflammation axis in malignant or metastatic cancers
Abstract
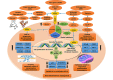
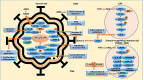
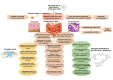
From attributing mutations to cancers with the advent of cutting-edge genetic technology in recent decades, to re-searching the age-old theory of intrinsic metabolic shift of cancers (Warburg's glycolysis), the quest for a precise panacea for mainly the metastatic cancers, remains incessant. This review delineates the advanced glycation end product (AGE)-receptor for AGE (RAGE) pathway driven intricate oncogenic cues, budding from the metabolic (glycolytic) reliance of tumour cells, branching into metastatic emergence of malignancies. Strong AGE-RAGE concomitance in metastasis, chemo-resistance and cancer resurgence adversely incite disease progression and patient mortality. At the conjunction of metabolic and metastatic shift of cancers, are the "glycolytically" generated AGEs and AGE-activated RAGE, instigating aberrant molecular pathways, culminating in aggressive malignancies. AGEs as by-products of metabolic insurgence, modify the metabolome, epigenome and microbiome, besides coercing the inter-, intra- and extra-cellular micro-milieu conducive for oncogenic events like epithelial-mesenchymal transition (EMT). AGE-RAGE synergistically elicit ATP surge for surplus energy, autophagy for apoptotic evasion and chemo-resistance, insulin-like growth factor 1 (IGF-1) for meta-inflammation and angiogenesis, high mobility group box-1 (HMGB1) for immune tolerance, S100 proteins for metastasis, and p53 protein attenuation for tumour suppression. AGEs are pronouncedly reported in invasive forms of breast, prostate, colon and pancreatic cancers, higher in patients with cancer than healthy counterparts, and higher in advanced stage than localized phase. Hence, the investigation of person-specific presence of AGEs, soluble RAGE and AGE-activated RAGE can be advocated as impending bio-markers for diagnostic, prognostic and therapeutic purposes, to predict cancer risk in patients with diabetes, obesity, metabolic syndrome as well as general population, to monitor prognosis and metastasis in patients with cancer, and to reckon complications in cancer survivors. Furthermore, clinical reports of exogenous (dietary) and endogenous (internally formed) AGEs in cancer patients, and contemporary clinical trials involving AGE-RAGE axis in cancer are underlined with theranostic implications.
Keywords: AGEs; RAGE; S100; cancer; epigenome; glycation; high mobility group box-1; microbiome.
© The Author(s) 2023.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
RAGE and Its Ligands: Molecular Interplay Between Glycation, Inflammation, and Hallmarks of Cancer-a Review.Horm Cancer. 2018 Oct;9(5):295-325. doi: 10.1007/s12672-018-0342-9. Epub 2018 Jul 9. Horm Cancer. 2018. PMID: 29987748 Free PMC article. Review.
-
Impact of Advanced Glycation End products (AGEs) and its receptor (RAGE) on cancer metabolic signaling pathways and its progression.Glycoconj J. 2021 Dec;38(6):717-734. doi: 10.1007/s10719-021-10031-x. Epub 2022 Jan 22. Glycoconj J. 2021. PMID: 35064413 Review.
-
Advanced Glycation End Products (AGEs), Glutathione and Breast Cancer: Factors, Mechanism and Therapeutic Interventions.Curr Drug Metab. 2019;20(1):65-71. doi: 10.2174/1389200219666180912104342. Curr Drug Metab. 2019. PMID: 30207227
-
The advanced glycation end-product Nϵ -carboxymethyllysine promotes progression of pancreatic cancer: implications for diabetes-associated risk and its prevention.J Pathol. 2018 Jun;245(2):197-208. doi: 10.1002/path.5072. Epub 2018 Apr 4. J Pathol. 2018. PMID: 29533466
-
Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives.Biomolecules. 2022 Apr 4;12(4):542. doi: 10.3390/biom12040542. Biomolecules. 2022. PMID: 35454131 Free PMC article. Review.
Cited by
-
Glycolipid Metabolic Disorders, Metainflammation, Oxidative Stress, and Cardiovascular Diseases: Unraveling Pathways.Biology (Basel). 2024 Jul 12;13(7):519. doi: 10.3390/biology13070519. Biology (Basel). 2024. PMID: 39056712 Free PMC article. Review.
-
Extracellular Interactors of the IGF System: Impact on Cancer Hallmarks and Therapeutic Approaches.Int J Mol Sci. 2024 May 29;25(11):5915. doi: 10.3390/ijms25115915. Int J Mol Sci. 2024. PMID: 38892104 Free PMC article. Review.
-
Advanced Glycation End-Products Acting as Immunomodulators for Chronic Inflammation, Inflammaging and Carcinogenesis in Patients with Diabetes and Immune-Related Diseases.Biomedicines. 2024 Jul 31;12(8):1699. doi: 10.3390/biomedicines12081699. Biomedicines. 2024. PMID: 39200164 Free PMC article. Review.
-
Glyoxalase System in Breast and Ovarian Cancers: Role of MEK/ERK/SMAD1 Pathway.Biomolecules. 2024 May 15;14(5):584. doi: 10.3390/biom14050584. Biomolecules. 2024. PMID: 38785990 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
