The combination of PD-L1 expression and the neutrophil-to-lymphocyte ratio as a prognostic factor of postoperative recurrence in non-small-cell lung cancer: a retrospective cohort study
- PMID: 37964220
- PMCID: PMC10644552
- DOI: 10.1186/s12885-023-11604-9
The combination of PD-L1 expression and the neutrophil-to-lymphocyte ratio as a prognostic factor of postoperative recurrence in non-small-cell lung cancer: a retrospective cohort study
Abstract
Background: While PD-L1 expression and neutrophil-to-lymphocyte ratio (NLR) are prognostic biomarkers for lung cancer, few studies have considered their interaction. We hypothesized that the product of PD-L1 expression (tumor proportion score) and the NLR (PD-L1 × NLR) might be a postoperative prognostic marker reflecting the immune microenvironment of lung cancer.
Methods: We analyzed the association between PD-L1 × NLR and postoperative recurrence-free survival in 647 patients with NSCLC using multivariable Cox proportional hazards models.
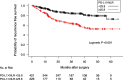
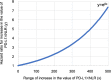
Results: In the analysis of PD-L1 × NLR as a categorical variable, the group with PD-L1 × NLR ≥ 25.8 had a significantly higher hazard ratio (HR) than the group with < 25.8 (adjusted HR 1.78, 95% confidence interval [CI] 1.23-2.60). The adjusted HR for PD-L1 × NLR, considered a continuous variable, was 1.004 (95% CI, 1.002-1.006). The risk of postoperative recurrence increased by 1.004-fold for each unit increase in PD-L1 × NLR, and a more than 2-fold increase in risk was observed for values ≥ 170.
Conclusions: PD-L1 × NLR may be used in real-world clinical practice as a novel factor for predicting the risk of postoperative recurrence after lung cancer surgery.
Keywords: Multivariable Cox proportional analysis; Neutrophil-to-lymphocyte ratio; Programmed death-ligand 1; Recurrence-free survival.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Prognostic impact of neutrophils-to-lymphocytes ratio (NLR), PD-L1 expression, and tumor immune microenvironment in laryngeal cancer.Ann Diagn Pathol. 2021 Feb;50:151657. doi: 10.1016/j.anndiagpath.2020.151657. Epub 2020 Nov 5. Ann Diagn Pathol. 2021. PMID: 33189034
-
Prognostic impact of PD-L1 expression in correlation with neutrophil-to-lymphocyte ratio in squamous cell carcinoma of the lung.Sci Rep. 2020 Jan 27;10(1):1243. doi: 10.1038/s41598-019-57321-x. Sci Rep. 2020. PMID: 31988315 Free PMC article.
-
Clinical value of neutrophil-to-lymphocyte ratio in patients with non-small-cell lung cancer treated with PD-1/PD-L1 inhibitors.Lung Cancer. 2019 Apr;130:76-83. doi: 10.1016/j.lungcan.2019.02.009. Epub 2019 Feb 10. Lung Cancer. 2019. PMID: 30885355
-
The Significance of the PD-L1 Expression in Non-Small-Cell Lung Cancer: Trenchant Double Swords as Predictive and Prognostic Markers.Clin Lung Cancer. 2018 Mar;19(2):120-129. doi: 10.1016/j.cllc.2017.10.014. Epub 2017 Oct 28. Clin Lung Cancer. 2018. PMID: 29153898 Review.
-
Neutrophil-to-Lymphocyte Ratio (NLR) in NSCLC, Gastrointestinal, and Other Solid Tumors: Immunotherapy and Beyond.Biomolecules. 2023 Dec 18;13(12):1803. doi: 10.3390/biom13121803. Biomolecules. 2023. PMID: 38136673 Free PMC article. Review.
Cited by
-
The Negative Impact of Inflammation-Related Parameters in Prostate Cancer after Robot-Assisted Radical Prostatectomy: A Retrospective Multicenter Cohort Study in Japan (the MSUG94 Group).J Clin Med. 2023 Dec 17;12(24):7732. doi: 10.3390/jcm12247732. J Clin Med. 2023. PMID: 38137801 Free PMC article.
-
A Review of the Prognostic Significance of Neutrophil-to-Lymphocyte Ratio in Nonhematologic Malignancies.Diagnostics (Basel). 2024 Sep 16;14(18):2057. doi: 10.3390/diagnostics14182057. Diagnostics (Basel). 2024. PMID: 39335736 Free PMC article. Review.
-
Nonlinear association between PD-L1 expression levels and the risk of postoperative recurrence in non-small cell lung cancer.Sci Rep. 2024 Jul 4;14(1):15369. doi: 10.1038/s41598-024-66463-6. Sci Rep. 2024. PMID: 38965343 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

