P2Y1R silencing in Astrocytes Protected Neuroinflammation and Cognitive Decline in a Mouse Model of Alzheimer's Disease
- PMID: 37962465
- PMCID: PMC11272185
- DOI: 10.14336/AD.2023.1006
P2Y1R silencing in Astrocytes Protected Neuroinflammation and Cognitive Decline in a Mouse Model of Alzheimer's Disease
Abstract
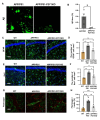
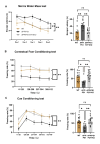
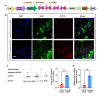
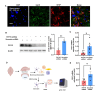
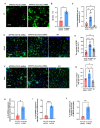
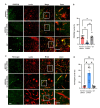
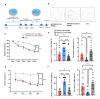
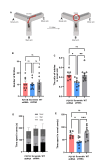
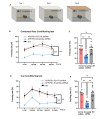
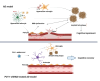
Astrocytes, the major non-dividing glial cells in the central nervous system, exhibit hyperactivation in Alzheimer's disease (AD), leading to neuroinflammation and cognitive impairments. P2Y1-receptor (P2Y1R) in AD brain has been pointed out some contribution to AD pathogenesis, therefore, this study aims to elucidate how astrocytic P2Y1R affects the progression of AD and explore its potential as a new target for AD therapy. In this study, we performed the two-steps verification to assess P2Y1R inhibition in AD progression: P2Y1R-KO AD mice and AD mice treated with astrocyte-specific P2Y1R gene knockdown by using shRNAs for P2Y1R in adeno-associated virus vector. Histochemistry was conducted for the assessment of amyloid-beta accumulation, neuroinflammation and blood brain barrier function. Expression of inflammatory cytokines was evaluated by qPCR after the separation of astrocytes. Cognitive function was assessed through the Morris water maze, Y maze, and contextual fear conditioning tests. P2Y1R inhibition not only by gene knockout but also by astrocyte-specific knockdown reduced amyloid-beta accumulation, glial neuroinflammation, blood brain barrier dysfunction, and cognitive impairment in an AD mice model. Reduced neuroinflammation by astrocytic P2Y1R silencing in AD was further confirmed by the reduction of IL-6 gene expression after the separation of astrocytes from AD mouse brain, which may relate to the amelioration of blood brain barrier as well as cognitive functions. Our results clearly note that P2Y1R in astrocyte contributes to the progression of AD pathology through the acceleration of neuroinflammation, and one-time gene therapy for silencing astrocytic P2Y1R may offer a new therapeutic target for AD.
Conflict of interest statement
The authors have no conflict of interest to report.
Figures
Similar articles
-
Genetic deletion of astrocytic calcineurin B1 prevents cognitive impairment and neuropathology development in acute and chronic mouse models of Alzheimer's disease.Glia. 2024 May;72(5):899-915. doi: 10.1002/glia.24509. Epub 2024 Jan 30. Glia. 2024. PMID: 38288580
-
P2Y1 receptor blockade normalizes network dysfunction and cognition in an Alzheimer's disease model.J Exp Med. 2018 Jun 4;215(6):1649-1663. doi: 10.1084/jem.20171487. Epub 2018 May 3. J Exp Med. 2018. PMID: 29724785 Free PMC article.
-
Adult-onset CNS myelin sulfatide deficiency is sufficient to cause Alzheimer's disease-like neuroinflammation and cognitive impairment.Mol Neurodegener. 2021 Sep 15;16(1):64. doi: 10.1186/s13024-021-00488-7. Mol Neurodegener. 2021. PMID: 34526055 Free PMC article.
-
Unveiling the role of astrocytes in postoperative cognitive dysfunction.Ageing Res Rev. 2024 Mar;95:102223. doi: 10.1016/j.arr.2024.102223. Epub 2024 Feb 5. Ageing Res Rev. 2024. PMID: 38325753 Review.
-
Calpain-Mediated Alterations in Astrocytes Before and During Amyloid Chaos in Alzheimer's Disease.J Alzheimers Dis. 2021;84(4):1415-1430. doi: 10.3233/JAD-215182. J Alzheimers Dis. 2021. PMID: 34719501 Review.
Cited by
-
Vascular Contributions to Healthy Aging and Dementia.Aging Dis. 2024 Aug 1;15(4):1432-1437. doi: 10.14336/AD.2023.1719. Aging Dis. 2024. PMID: 39059424 Free PMC article.
References
-
- Lozupone M, Solfrizzi V, D'Urso F, Di Gioia I, Sardone R, Dibello V, Stallone R, Liguori A, Ciritella C, Daniele A, Bellomo A, Seripa D, Panza F (2020). Anti-amyloid-beta protein agents for the treatment of Alzheimer's disease: an update on emerging drugs. Expert Opin Emerg Drugs, 25:319-335. - PubMed
-
- Allen NJ (2013). Role of glia in developmental synapse formation. Curr Opin Neurobiol, 23: 1027-1033. - PubMed
-
- Han RT, Kim RD, Molofsky AV, Liddelow SA (2021). Astrocyte-immune cell interactions in physiology and pathology. Immunity, 54:211-224. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
