Effects of Aeriscardovia aeriphila on growth performance, antioxidant functions, immune responses, and gut microbiota in broiler chickens
- PMID: 37961803
- PMCID: PMC10646399
- DOI: 10.1631/jzus.B2200621
Effects of Aeriscardovia aeriphila on growth performance, antioxidant functions, immune responses, and gut microbiota in broiler chickens
Abstract
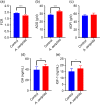
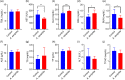
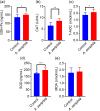
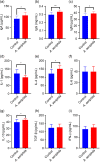
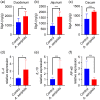
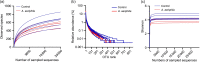
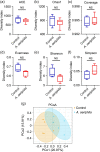
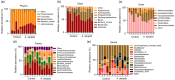
Aeriscardovia aeriphila, also known as Bifidobacterium aerophilum, was first isolated from the caecal contents of pigs and the faeces of cotton-top tamarin. Bifidobacterium species play important roles in preventing intestinal infections, decreasing cholesterol levels, and stimulating the immune system. In this study, we isolated a strain of bacteria from the duodenal contents of broiler chickens, which was identified as A. aeriphila, and then evaluated the effects of A. aeriphila on growth performance, antioxidant functions, immune functions, and gut microbiota in commercial broiler chickens. Chickens were orally gavaged with A. aeriphila (1×109 CFU/mL) for 21 d. The results showed that A. aeriphila treatment significantly increased the average daily gain and reduced the feed conversion ratio (P<0.001). The levels of serum growth hormone (GH) and insulin-like growth factor 1 (IGF-1) were significantly increased following A. aeriphila treatment (P<0.05). Blood urea nitrogen and aspartate aminotransferase levels were decreased, whereas glucose and creatinine levels increased as a result of A. aeriphila treatment. Furthermore, the levels of serum antioxidant enzymes, including catalase (P<0.01), superoxide dismutase (P<0.001), and glutathione peroxidase (P<0.05), and total antioxidant capacity (P<0.05) were enhanced following A. aeriphila treatment. A. aeriphila treatment significantly increased the levels of serum immunoglobulin A (IgA) (P<0.05), IgG (P<0.01), IgM (P<0.05), interleukin-1 (IL-1) (P<0.05), IL-4 (P<0.05), and IL-10 (P<0.05). The broiler chickens in the A. aeriphila group had higher secretory IgA (SIgA) levels in the duodenum (P<0.01), jejunum (P<0.001), and cecum (P<0.001) than those in the control group. The messenger RNA (mRNA) relative expression levels of IL-10 (P<0.05) and IL-4 (P<0.001) in the intestinal mucosa of chickens were increased, while nuclear factor-κB (NF-κB) (P<0.001) expression was decreased in the A. aeriphila group compared to the control group. Phylum-level analysis revealed Firmicutes as the main phylum, followed by Bacteroidetes, in both groups. The data also found that Phascolarctobacterium and Barnesiella were increased in A. aeriphila-treated group. In conclusion, oral administration of A. aeriphila could improve the growth performance, serum antioxidant capacity, immune modulation, and gut health of broilers. Our findings may provide important information for the application of A. aeriphila in poultry production.
Aeriscardovia aeriphila,又名Bifidobacterium aerophilum,是从猪盲肠内容物中分离出的一种双歧杆菌。A. aeriphila在预防肠道感染、降低胆固醇水平和刺激免疫系统方面具有重要作用。本研究首先从肉鸡十二指肠肠道内容物中分离出一株菌, 经16S 测序鉴定为 A. aeriphila,然后我们评估了A. aeriphila对商用肉鸡生长性能、抗氧化功能、免疫功能和肠道菌群的影响。实验对21日龄的实验鸡进行了为期3周的灌服A. aeriphila(1×109 CFU/mL)处理。研究结果显示:A. aeriphila处理显著增加了鸡的平均日增重,降低了饲料转化率(P<0.001)。A. aeriphila处理后,血清生长激素(GH)和胰岛素样生长因子1(IGF-1)水平显著升高(P<0.05)。A. aeriphila处理降低了血尿素氮和天门冬氨酸氨基转移酶水平,增加了血糖和肌酐水平。同时,A. aeriphila处理显著提高了血清中抗氧化酶水平(包括过氧化氢酶(P<0.01)、超氧化物歧化酶(P<0.001)和谷胱甘肽过氧化物酶(P<0.05))及总抗氧化能力(P<0.05)。A. aeriphila处理显著增加了血清免疫球蛋白A(IgA)(P<0.05)、IgG(P<0.01)、IgM(P<0.05)、白细胞介素-1(IL-1)(P<0.05)、IL-4(P<0.05)和IL-10(P<0.05)的水平。与对照组相比,A. aeriphila组肉鸡在十二指肠(P<0.01)、空肠(P<0.001)和盲肠(P<0.001)中的分泌型免疫球蛋白A(SIgA)水平显著提高。在肠道黏膜(包括十二指肠、空肠和盲肠)中,A. aeriphila组鸡的IL-10(P<0.05)、IL-4(P<0.001)和NF-κB(P<0.001)信使RNA(mRNA)相对表达水平高于对照组。肠道微生物门水平分析显示,两组的差异菌群主要是厚壁菌门,其次是拟杆菌门。此外,A. aeriphila可提高肠道中Phascolarctobacterium和Barnesiella的丰度。综上所述,灌服A. aeriphila可改善肉鸡的生长性能、血清抗氧化能力、免疫调节和肠道健康。因此,本研究结果可以为A. aeriphila在家禽生产中的应用提供理论依据。.
Aeriscardovia aeriphila,又名 Bifidobacterium aerophilum,是从猪盲肠内容物中分离出的一种双歧杆菌。 A. aeriphila在预防肠道感染、降低胆固醇水平和刺激免疫系统方面具有重要作用。本研究首先从肉鸡十二指肠肠道内容物中分离出一株菌, 经16S 测序鉴定为 A. aeriphila,然后我们评估了 A. aeriphila对商用肉鸡生长性能、抗氧化功能、免疫功能和肠道菌群的影响。实验对21日龄的实验鸡进行了为期3周的灌服 A. aeriphila(1×10 9 CFU/mL)处理。研究结果显示: A. aeriphila处理显著增加了鸡的平均日增重,降低了饲料转化率( P<0.001)。 A. aeriphila处理后,血清生长激素(GH)和胰岛素样生长因子1(IGF-1)水平显著升高( P<0.05)。 A. aeriphila处理降低了血尿素氮和天门冬氨酸氨基转移酶水平,增加了血糖和肌酐水平。同时, A. aeriphila处理显著提高了血清中抗氧化酶水平(包括过氧化氢酶( P<0.01)、超氧化物歧化酶( P<0.001)和谷胱甘肽过氧化物酶( P<0.05))及总抗氧化能力( P<0.05)。 A. aeriphila处理显著增加了血清免疫球蛋白A(IgA)( P<0.05)、IgG( P<0.01)、IgM( P<0.05)、白细胞介素-1(IL-1)( P<0.05)、IL-4( P<0.05)和IL-10( P<0.05)的水平。与对照组相比, A. aeriphila组肉鸡在十二指肠( P<0.01)、空肠( P<0.001)和盲肠( P<0.001)中的分泌型免疫球蛋白A(SIgA)水平显著提高。在肠道黏膜(包括十二指肠、空肠和盲肠)中, A. aeriphila组鸡的 IL-10( P<0.05)、 IL-4( P<0.001)和 NF-κB( P<0.001)信使RNA(mRNA)相对表达水平高于对照组。肠道微生物门水平分析显示,两组的差异菌群主要是厚壁菌门,其次是拟杆菌门。此外, A. aeriphila可提高肠道中 Phascolarctobacterium和 Barnesiella的丰度。综上所述,灌服 A. aeriphila可改善肉鸡的生长性能、血清抗氧化能力、免疫调节和肠道健康。因此,本研究结果可以为 A. aeriphila在家禽生产中的应用提供理论依据。
Keywords: Aeriscardovia aeriphila; Chicken; Gut health; Immune function; Microbiome.
Figures
Similar articles
-
Dietary Chinese herbal mixture supplementation improves production performance by regulating reproductive hormones, antioxidant capacity, immunity, and intestinal health of broiler breeders.Poult Sci. 2024 Jan;103(1):103201. doi: 10.1016/j.psj.2023.103201. Epub 2023 Oct 21. Poult Sci. 2024. PMID: 37980727 Free PMC article.
-
Effects of bamboo leaf flavonoids on growth performance, antioxidants, immune function, intestinal morphology, and cecal microbiota in broilers.J Sci Food Agric. 2024 Sep;104(12):7656-7667. doi: 10.1002/jsfa.13602. Epub 2024 May 21. J Sci Food Agric. 2024. PMID: 38770921
-
Selenium-enriched Bacillus subtilis Improves Growth Performance, Antioxidant Capacity, Immune Status, and Gut Health of Broiler Chickens.Biol Trace Elem Res. 2023 Dec;201(12):5756-5763. doi: 10.1007/s12011-023-03610-6. Epub 2023 Mar 2. Biol Trace Elem Res. 2023. PMID: 36862247
-
Antioxidant Indexes and Immune Function of the Intestinal Flora of Compound Microecological Preparations.Oxid Med Cell Longev. 2022 Sep 30;2022:5498514. doi: 10.1155/2022/5498514. eCollection 2022. Oxid Med Cell Longev. 2022. Retraction in: Oxid Med Cell Longev. 2024 Jan 9;2024:9780381. doi: 10.1155/2024/9780381. PMID: 36238651 Free PMC article. Retracted. Review.
-
The Impact of Essential Amino Acids on the Gut Microbiota of Broiler Chickens.Microorganisms. 2024 Mar 29;12(4):693. doi: 10.3390/microorganisms12040693. Microorganisms. 2024. PMID: 38674637 Free PMC article. Review.
Cited by
-
Influence of Varied Environment Conditions on the Gut Microbiota of Yaks.Animals (Basel). 2024 May 25;14(11):1570. doi: 10.3390/ani14111570. Animals (Basel). 2024. PMID: 38891617 Free PMC article.
References
-
- Alaqaby ARA, Glaskovich AA, Kapitonova EA, et al. , 2014. Study the effect of using probiotic (vetlactoflorum) on some of biochemical and immunological parameters of broiler chickens. Basrah J Vet Res, 13( 1): 166- 179. 10.33762/bvetr.2014.88137 - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

