This is a preprint.
The membrane-associated ubiquitin ligase MARCHF8 stabilizes the human papillomavirus oncoprotein E7 by degrading CUL1 and UBE2L3 in head and neck cancer
- PMID: 37961092
- PMCID: PMC10635129
- DOI: 10.1101/2023.11.03.565564
The membrane-associated ubiquitin ligase MARCHF8 stabilizes the human papillomavirus oncoprotein E7 by degrading CUL1 and UBE2L3 in head and neck cancer
Update in
-
The membrane-associated ubiquitin ligase MARCHF8 stabilizes the human papillomavirus oncoprotein E7 by degrading CUL1 and UBE2L3 in head and neck cancer.J Virol. 2024 Feb 20;98(2):e0172623. doi: 10.1128/jvi.01726-23. Epub 2024 Jan 16. J Virol. 2024. PMID: 38226814 Free PMC article.
Abstract
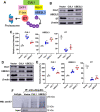
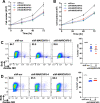
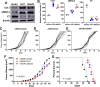
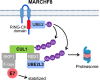
The human papillomavirus (HPV) oncoprotein E7 is a relatively short-lived protein required for HPV-driven cancer development and maintenance. E7 is degraded through ubiquitination mediated by cullin 1 (CUL1) and the ubiquitin-conjugating enzyme E2 L3 (UBE2L3). However, E7 proteins are maintained at high levels in most HPV-positive cancer cells. A previous proteomics study has shown that UBE2L3 and CUL1 protein levels are increased by the knockdown of the E3 ubiquitin ligase membrane-associated ring-CH-type finger 8 (MARCHF8). We have recently demonstrated that HPV upregulates MARCHF8 expression in HPV-positive keratinocytes and head and neck cancer (HPV+ HNC) cells. Here, we report that MARCHF8 stabilizes the E7 protein by degrading the components of the SKP1-CUL1-F-box (SCF) ubiquitin ligase complex in HPV+ HNC cells. We found that MARCHF8 knockdown in HPV+ HNC cells drastically decreases the E7 protein level while increasing the CUL1 and UBE2L3 protein levels. We further revealed that the MARCHF8 protein binds to and ubiquitinates CUL1 and UBE2L3 proteins and that MARCHF8 knockdown enhances the ubiquitination of the E7 protein. Conversely, the overexpression of CUL1 and UBE2L3 in HPV+ HNC cells decreases E7 protein levels and suppresses tumor growth in vivo. Our findings suggest that HPV-induced MARCHF8 prevents the degradation of the E7 protein in HPV+ HNC cells by ubiquitinating and degrading CUL1 and UBE2L3 proteins.
Figures




Similar articles
-
The membrane-associated ubiquitin ligase MARCHF8 stabilizes the human papillomavirus oncoprotein E7 by degrading CUL1 and UBE2L3 in head and neck cancer.J Virol. 2024 Feb 20;98(2):e0172623. doi: 10.1128/jvi.01726-23. Epub 2024 Jan 16. J Virol. 2024. PMID: 38226814 Free PMC article.
-
HPV upregulates MARCHF8 ubiquitin ligase and inhibits apoptosis by degrading the death receptors in head and neck cancer.PLoS Pathog. 2023 Mar 3;19(3):e1011171. doi: 10.1371/journal.ppat.1011171. eCollection 2023 Mar. PLoS Pathog. 2023. PMID: 36867660 Free PMC article.
-
The papillomavirus E7 oncoprotein is ubiquitinated by UbcH7 and Cullin 1- and Skp2-containing E3 ligase.J Virol. 2004 May;78(10):5338-46. doi: 10.1128/jvi.78.10.5338-5346.2004. J Virol. 2004. PMID: 15113913 Free PMC article.
-
Human Papillomavirus 16 E7 Stabilizes APOBEC3A Protein by Inhibiting Cullin 2-Dependent Protein Degradation.J Virol. 2018 Mar 14;92(7):e01318-17. doi: 10.1128/JVI.01318-17. Print 2018 Apr 1. J Virol. 2018. PMID: 29367246 Free PMC article.
-
Genetically engineered mouse models for functional studies of SKP1-CUL1-F-box-protein (SCF) E3 ubiquitin ligases.Cell Res. 2013 May;23(5):599-619. doi: 10.1038/cr.2013.44. Epub 2013 Mar 26. Cell Res. 2013. PMID: 23528706 Free PMC article. Review.
References
-
- Pyeon D, Newton MA, Lambert PF, den Boon JA, Sengupta S, Marsit CJ, Woodworth CD, Connor JP, Haugen TH, Smith EM, Kelsey KT, Turek LP, Ahlquist P. 2007. Fundamental differences in cell cycle deregulation in human papillomavirus-positive and human papillomavirus-negative head/neck and cervical cancers. Cancer Res 67:4605–19. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
