The Dysfunction of Ca2+ Channels in Hereditary and Chronic Human Heart Diseases and Experimental Animal Models
- PMID: 37958665
- PMCID: PMC10650855
- DOI: 10.3390/ijms242115682
The Dysfunction of Ca2+ Channels in Hereditary and Chronic Human Heart Diseases and Experimental Animal Models
Abstract
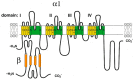
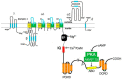
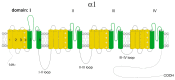
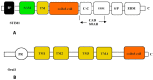
Chronic heart diseases, such as coronary heart disease, heart failure, secondary arterial hypertension, and dilated and hypertrophic cardiomyopathies, are widespread and have a fairly high incidence of mortality and disability. Most of these diseases are characterized by cardiac arrhythmias, conduction, and contractility disorders. Additionally, interruption of the electrical activity of the heart, the appearance of extensive ectopic foci, and heart failure are all symptoms of a number of severe hereditary diseases. The molecular mechanisms leading to the development of heart diseases are associated with impaired permeability and excitability of cell membranes and are mainly caused by the dysfunction of cardiac Ca2+ channels. Over the past 50 years, more than 100 varieties of ion channels have been found in the cardiovascular cells. The relationship between the activity of these channels and cardiac pathology, as well as the general cellular biological function, has been intensively studied on several cell types and experimental animal models in vivo and in situ. In this review, I discuss the origin of genetic Ca2+ channelopathies of L- and T-type voltage-gated calcium channels in humans and the role of the non-genetic dysfunctions of Ca2+ channels of various types: L-, R-, and T-type voltage-gated calcium channels, RyR2, including Ca2+ permeable nonselective cation hyperpolarization-activated cyclic nucleotide-gated (HCN), and transient receptor potential (TRP) channels, in the development of cardiac pathology in humans, as well as various aspects of promising experimental studies of the dysfunctions of these channels performed on animal models or in vitro.
Keywords: HCN channels; RyR2; TRP channels; animal model; calcium channelopathies; cardiac arrhythmias; cardiac calcium channels; gene regulation; heart diseases; knockout model.
Conflict of interest statement
The author declares no conflict of interest.
Figures
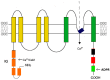
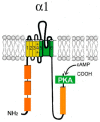
Similar articles
-
TRP Channels in the Heart.In: Emir TLR, editor. Neurobiology of TRP Channels. Boca Raton (FL): CRC Press/Taylor & Francis; 2017. Chapter 9. In: Emir TLR, editor. Neurobiology of TRP Channels. Boca Raton (FL): CRC Press/Taylor & Francis; 2017. Chapter 9. PMID: 29356479 Free Books & Documents. Review.
-
HCN-related channelopathies.Pflugers Arch. 2010 Jul;460(2):405-15. doi: 10.1007/s00424-010-0810-8. Epub 2010 Mar 8. Pflugers Arch. 2010. PMID: 20213494 Review.
-
Direct evidence for calcium conductance of hyperpolarization-activated cyclic nucleotide-gated channels and human native If at physiological calcium concentrations.Cardiovasc Res. 2008 Jun 1;78(3):466-75. doi: 10.1093/cvr/cvn032. Epub 2008 Feb 5. Cardiovasc Res. 2008. PMID: 18252758
-
Review: HCN Channels in the Heart.Curr Cardiol Rev. 2022;18(4):e040222200836. doi: 10.2174/1573403X18666220204142436. Curr Cardiol Rev. 2022. PMID: 35125083 Free PMC article. Review.
-
Cardiac and neuronal HCN channelopathies.Pflugers Arch. 2020 Jul;472(7):931-951. doi: 10.1007/s00424-020-02384-3. Epub 2020 May 18. Pflugers Arch. 2020. PMID: 32424620 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

