Evaluation of the Immunoprotective Capacity of Five Vaccine Candidate Proteins against Avian Necrotic Enteritis and Impact on the Caecal Microbiota of Vaccinated Birds
- PMID: 37958078
- PMCID: PMC10650611
- DOI: 10.3390/ani13213323
Evaluation of the Immunoprotective Capacity of Five Vaccine Candidate Proteins against Avian Necrotic Enteritis and Impact on the Caecal Microbiota of Vaccinated Birds
Abstract
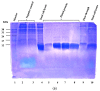
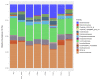
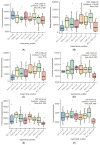
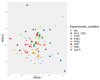
Avian necrotic enteritis is an enteric disease of broiler chickens caused by certain pathogenic strains of Clostridium perfringens in combination with predisposing factors. A vaccine offering complete protection against the disease has not yet been commercialized. In a previous study, we produced five recombinant proteins predicted to be surface-exposed and unique to necrotic enteritis-causing C. perfringens and the immunogenicity of these potential vaccine candidates was assessed in broiler chickens. In the current work, the relative contribution of the antibodies raised by these putative antigens to protect broiler chickens was evaluated using an experimental necrotic enteritis induction model. Additionally, the link between the immune response elicited and the gut microbiota profiles in immunized birds subjected to infection with virulent C. perfringens was studied. The ELISA results showed that the IgY antibody titers in vaccinated birds on days 21 and 33 were significantly higher than those on days 7 and 14 and those in birds receiving the adjuvant alone, while the relative contribution of the specific immunity attributed to these antibodies could not be precisely determined using this experimental necrotic enteritis induction model. In addition, 16S rRNA gene amplicon sequencing showed that immunization of birds with recombinant proteins had a low impact on the chicken caecal microbiota.
Keywords: Clostridium perfringens; broiler chickens; gut microbiota; immune response; necrotic enteritis; surface-exposed antigenic proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Vaccination with Clostridium perfringens recombinant proteins in combination with Montanide™ ISA 71 VG adjuvant increases protection against experimental necrotic enteritis in commercial broiler chickens.Vaccine. 2012 Aug 3;30(36):5401-6. doi: 10.1016/j.vaccine.2012.06.007. Epub 2012 Jun 17. Vaccine. 2012. PMID: 22713719
-
Lactobacillus casei displaying Clostridium perfringens NetB antigen protects chickens against necrotic enteritis.Appl Microbiol Biotechnol. 2022 Oct;106(19-20):6441-6453. doi: 10.1007/s00253-022-12155-y. Epub 2022 Sep 5. Appl Microbiol Biotechnol. 2022. PMID: 36063180
-
Immunization of broiler chickens against Clostridium perfringens-induced necrotic enteritis.Clin Vaccine Immunol. 2007 Sep;14(9):1070-7. doi: 10.1128/CVI.00162-07. Epub 2007 Jul 18. Clin Vaccine Immunol. 2007. PMID: 17634510 Free PMC article.
-
Necrotic enteritis predisposing factors in broiler chickens.Avian Pathol. 2016 Jun;45(3):275-81. doi: 10.1080/03079457.2016.1150587. Avian Pathol. 2016. PMID: 26926926 Review.
-
Necrotic enteritis in broilers: an updated review on the pathogenesis.Avian Pathol. 2011 Aug;40(4):341-7. doi: 10.1080/03079457.2011.590967. Avian Pathol. 2011. PMID: 21812711 Review.
Cited by
-
Identification of immunogenic antigens and evaluation of vaccine candidates against Clostridium perfringens.Poult Sci. 2024 Dec;103(12):104436. doi: 10.1016/j.psj.2024.104436. Epub 2024 Oct 13. Poult Sci. 2024. PMID: 39467405 Free PMC article.
References
-
- Diaz Carrasco J.M., Redondo L.M., Redondo E.A., Dominguez J.E., Chacana A.P., Fernandez Miyakawa M.E. Use of plant extracts as an effective manner to control Clostridium perfringens induced necrotic enteritis in poultry. Biomed. Res. Int. 2016;2016:3278359. doi: 10.1155/2016/3278359. - DOI - PMC - PubMed
-
- Scott K.P., Gratz S.W., Sheridan P.O., Flint H.J., Duncan S.H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013;69:52–60. - PubMed
-
- Lacey J.A., Keyburn A.L., Ford M.E., Portela R.W., Johanesen P.A., Lyras D., Moore R.J. Conjugation-mediated horizontal gene transfer of Clostridium perfringens plasmids in the chicken gastrointestinal tract results in the formation of new virulent strains. Appl. Environ. Microbiol. 2017;83:e01814-17. doi: 10.1128/AEM.01814-17. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

