Menstrual blood-derived endometrial stem cell, a unique and promising alternative in the stem cell-based therapy for chemotherapy-induced premature ovarian insufficiency
- PMID: 37957675
- PMCID: PMC10644549
- DOI: 10.1186/s13287-023-03551-w
Menstrual blood-derived endometrial stem cell, a unique and promising alternative in the stem cell-based therapy for chemotherapy-induced premature ovarian insufficiency
Abstract
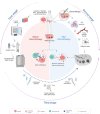
Chemotherapy can cause ovarian dysfunction and infertility since the ovary is extremely sensitive to chemotherapeutic drugs. Apart from the indispensable role of the ovary in the overall hormonal milieu, ovarian dysfunction also affects many other organ systems and functions including sexuality, bones, the cardiovascular system, and neurocognitive function. Although conventional hormone replacement therapy can partly relieve the adverse symptoms of premature ovarian insufficiency (POI), the treatment cannot fundamentally prevent deterioration of POI. Therefore, effective treatments to improve chemotherapy-induced POI are urgently needed, especially for patients desiring fertility preservation. Recently, mesenchymal stem cell (MSC)-based therapies have resulted in promising improvements in chemotherapy-induced ovary dysfunction by enhancing the anti-apoptotic capacity of ovarian cells, preventing ovarian follicular atresia, promoting angiogenesis and improving injured ovarian structure and the pregnancy rate. These improvements are mainly attributed to MSC-derived biological factors, functional RNAs, and even mitochondria, which are directly secreted or indirectly translocated with extracellular vesicles (microvesicles and exosomes) to repair ovarian dysfunction. Additionally, as a novel source of MSCs, menstrual blood-derived endometrial stem cells (MenSCs) have exhibited promising therapeutic effects in various diseases due to their comprehensive advantages, such as periodic and non-invasive sample collection, abundant sources, regular donation and autologous transplantation. Therefore, this review summarizes the efficacy of MSCs transplantation in improving chemotherapy-induced POI and analyzes the underlying mechanism, and further discusses the benefit and existing challenges in promoting the clinical application of MenSCs in chemotherapy-induced POI.
Keywords: Chemotherapy; Fertility; Menstrual blood-derived endometrial stem cells; Mesenchymal stem cells; Paracrine effect; Premature ovarian insufficiency.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation improves ovarian function in rats with premature ovarian insufficiency (POI) at least partly through a paracrine mechanism.Stem Cell Res Ther. 2019 Jan 25;10(1):46. doi: 10.1186/s13287-019-1136-x. Stem Cell Res Ther. 2019. PMID: 30683144 Free PMC article.
-
Comparison of the therapeutic effects between stem cells and exosomes in primary ovarian insufficiency: as promising as cells but different persistency and dosage.Stem Cell Res Ther. 2023 Jun 20;14(1):165. doi: 10.1186/s13287-023-03397-2. Stem Cell Res Ther. 2023. PMID: 37340468 Free PMC article.
-
Effects of Low-Intensity Pulsed Ultrasound on the Migration and Homing of Human Amnion-Derived Mesenchymal Stem Cells to Ovaries in Rats With Premature Ovarian Insufficiency.Cell Transplant. 2022 Jan-Dec;31:9636897221129171. doi: 10.1177/09636897221129171. Cell Transplant. 2022. PMID: 36282038 Free PMC article.
-
Premature ovarian insufficiency: pathogenesis and therapeutic potential of mesenchymal stem cell.J Mol Med (Berl). 2021 May;99(5):637-650. doi: 10.1007/s00109-021-02055-5. Epub 2021 Feb 27. J Mol Med (Berl). 2021. PMID: 33641066 Review.
-
Human UC-MSC-derived exosomes facilitate ovarian renovation in rats with chemotherapy-induced premature ovarian insufficiency.Front Endocrinol (Lausanne). 2023 Jul 26;14:1205901. doi: 10.3389/fendo.2023.1205901. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37564988 Free PMC article. Review.
Cited by
-
Evolution of biotechnological advances and regenerative therapies for endometrial disorders: a systematic review.Hum Reprod Update. 2024 Oct 1;30(5):584-613. doi: 10.1093/humupd/dmae013. Hum Reprod Update. 2024. PMID: 38796750 Free PMC article.
-
Therapeutic potential of endometrial stem cells encapsulated in alginate/gelatin hydrogel to treat of polycystic ovary syndrome.Regen Ther. 2024 Aug 30;26:693-707. doi: 10.1016/j.reth.2024.08.016. eCollection 2024 Jun. Regen Ther. 2024. PMID: 39286642 Free PMC article.
-
The clinical value of acupuncture for women with premature ovarian insufficiency: a systematic review and meta-analysis of randomized controlled trials.Front Endocrinol (Lausanne). 2024 Jul 11;15:1361573. doi: 10.3389/fendo.2024.1361573. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39055062 Free PMC article.
-
Menstrual Blood Stem Cells-Derived Exosomes as Promising Therapeutic Tools in Premature Ovarian Insufficiency Induced by Gonadotoxic Systemic Anticancer Treatment.Int J Mol Sci. 2024 Aug 2;25(15):8468. doi: 10.3390/ijms25158468. Int J Mol Sci. 2024. PMID: 39126037 Free PMC article. Review.
References
-
- van Dorp W, Haupt R, Anderson RA, Mulder RL, van den Heuvel-Eibrink MM, van Dulmen-den BE, et al. Reproductive function and outcomes in female survivors of childhood, adolescent, and young adult cancer: a review. J Clin Oncol. 2018;36(21):2169–2180. doi: 10.1200/JCO.2017.76.3441. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

