The mechanisms of action of mitochondrial targeting agents in cancer: inhibiting oxidative phosphorylation and inducing apoptosis
- PMID: 37954849
- PMCID: PMC10635426
- DOI: 10.3389/fphar.2023.1243613
The mechanisms of action of mitochondrial targeting agents in cancer: inhibiting oxidative phosphorylation and inducing apoptosis
Abstract
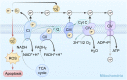
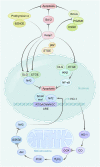
The tumor microenvironment affects the structure and metabolic function of mitochondria in tumor cells. This process involves changes in metabolic activity, an increase in the amount of reactive oxygen species (ROS) in tumor cells compared to normal cells, the production of more intracellular free radicals, and the activation of oxidative pathways. From a practical perspective, it is advantageous to develop drugs that target mitochondria for the treatment of malignant tumors. Such drugs can enhance the selectivity of treatments for specific cell groups, minimize toxic effects on normal tissues, and improve combinational treatments. Mitochondrial targeting agents typically rely on small molecule medications (such as synthetic small molecules agents, active ingredients of plants, mitochondrial inhibitors or autophagy inhibitors, and others), modified mitochondrial delivery system agents (such as lipophilic cation modification or combining other molecules to form targeted mitochondrial agents), and a few mitochondrial complex inhibitors. This article will review these compounds in three main areas: oxidative phosphorylation (OXPHOS), changes in ROS levels, and endogenous oxidative and apoptotic processes.
Keywords: electron transport chain (ETC); mechanism; mitocans; mitochondria targeting drug; oxidative phoshorylation; reactive oxygen species.
Copyright © 2023 Yang, An, Ren, Wang, Bai, Du and Kong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
OXPHOS-targeting drugs in oncology: new perspectives.Expert Opin Ther Targets. 2023 Jul-Dec;27(10):939-952. doi: 10.1080/14728222.2023.2261631. Epub 2023 Oct 30. Expert Opin Ther Targets. 2023. PMID: 37736880 Free PMC article. Review.
-
Mitocans: mitochondrial targeted anti-cancer drugs as improved therapies and related patent documents.Recent Pat Anticancer Drug Discov. 2006 Nov;1(3):327-46. doi: 10.2174/157489206778776952. Recent Pat Anticancer Drug Discov. 2006. PMID: 18221044 Review.
-
Exploiting Mitochondrial Vulnerabilities to Trigger Apoptosis Selectively in Cancer Cells.Cancers (Basel). 2019 Jun 29;11(7):916. doi: 10.3390/cancers11070916. Cancers (Basel). 2019. PMID: 31261935 Free PMC article. Review.
-
Tumor cell death induced by the inhibition of mitochondrial electron transport: the effect of 3-hydroxybakuchiol.Toxicol Appl Pharmacol. 2013 Oct 15;272(2):356-64. doi: 10.1016/j.taap.2013.06.005. Epub 2013 Jun 15. Toxicol Appl Pharmacol. 2013. PMID: 23777606
-
Negative modulation of mitochondrial oxidative phosphorylation by epigallocatechin-3 gallate leads to growth arrest and apoptosis in human malignant pleural mesothelioma cells.Biochim Biophys Acta. 2013 Dec;1832(12):2085-96. doi: 10.1016/j.bbadis.2013.07.014. Epub 2013 Aug 2. Biochim Biophys Acta. 2013. PMID: 23911347
Cited by
-
Combining Photodynamic Therapy and Targeted Drug Delivery Systems: Enhancing Mitochondrial Toxicity for Improved Cancer Outcomes.Int J Mol Sci. 2024 Oct 8;25(19):10796. doi: 10.3390/ijms251910796. Int J Mol Sci. 2024. PMID: 39409125 Free PMC article. Review.
-
Photoactivated hydride therapy under hypoxia beyond ROS.Chem Sci. 2024 Nov 12;15(48):20292-20302. doi: 10.1039/d4sc06576j. eCollection 2024 Dec 11. Chem Sci. 2024. PMID: 39568933 Free PMC article.
-
Kaempferol Synergistically Enhances Cisplatin-induced Apoptosis and Cell Cycle Arrest in Colon Cancer Cells.J Cancer Prev. 2024 Sep 30;29(3):69-87. doi: 10.15430/JCP.24.013. J Cancer Prev. 2024. PMID: 39398110 Free PMC article.
-
Anti-Cancer Potential of Isoflavone-Enriched Fraction from Traditional Thai Fermented Soybean against Hela Cervical Cancer Cells.Int J Mol Sci. 2024 Aug 27;25(17):9277. doi: 10.3390/ijms25179277. Int J Mol Sci. 2024. PMID: 39273231 Free PMC article.
-
ULK1 Mediated Autophagy-Promoting Effects of Rutin-Loaded Chitosan Nanoparticles Contribute to the Activation of NF-κB Signaling Besides Inhibiting EMT in Hep3B Hepatoma Cells.Int J Nanomedicine. 2024 May 18;19:4465-4493. doi: 10.2147/IJN.S443117. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38779103 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources