Infiltrating myeloid cell-derived properdin markedly promotes microglia-mediated neuroinflammation after ischemic stroke
- PMID: 37951917
- PMCID: PMC10640761
- DOI: 10.1186/s12974-023-02946-z
Infiltrating myeloid cell-derived properdin markedly promotes microglia-mediated neuroinflammation after ischemic stroke
Abstract
Background: Emerging evidence has shown that myeloid cells that infiltrate into the peri-infarct region may influence the progression of ischemic stroke by interacting with microglia. Properdin, which is typically secreted by immune cells such as neutrophils, monocytes, and T cells, has been found to possess damage-associated molecular patterns (DAMPs) properties and can perform functions unrelated to the complement pathway. However, the role of properdin in modulating microglia-mediated post-stroke neuroinflammation remains unclear.
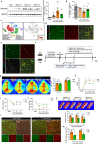
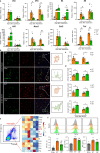
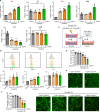
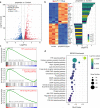
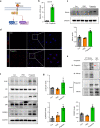
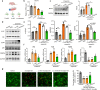
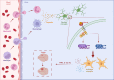
Methods: Global and conditional (myeloid-specific) properdin-knockout mice were subjected to transient middle cerebral artery occlusion (tMCAO). Histopathological and behavioral tests were performed to assess ischemic brain injury in mice. Single-cell RNA sequencing and immunofluorescence staining were applied to explore the source and the expression level of properdin. The transcriptomic profile of properdin-activated primary microglia was depicted by transcriptome sequencing. Lentivirus was used for macrophage-inducible C-type lectin (Mincle) silencing in microglia. Conditioned medium from primary microglia was administered to primary cortex neurons to determine the neurotoxicity of microglia. A series of cellular and molecular biological techniques were used to evaluate the proinflammatory response, neuronal death, protein-protein interactions, and related signaling pathways, etc. RESULTS: The level of properdin was significantly increased, and brain-infiltrating neutrophils and macrophages were the main sources of properdin in the ischemic brain. Global and conditional myeloid knockout of properdin attenuated microglial overactivation and inflammatory responses at the acute stage of tMCAO in mice. Accordingly, treatment with recombinant properdin enhanced the production of proinflammatory cytokines and augmented microglia-potentiated neuronal death in primary culture. Mechanistically, recombinant properdin served as a novel ligand that activated Mincle receptors on microglia and downstream pathways to drive primary microglia-induced inflammatory responses. Intriguingly, properdin can directly bind to the microglial Mincle receptor to exert the above effects, while Mincle knockdown limits properdin-mediated microglial inflammation.
Conclusion: Properdin is a new medium by which infiltrating peripheral myeloid cells communicate with microglia, further activate microglia, and exacerbate brain injury in the ischemic brain, suggesting that targeted disruption of the interaction between properdin and Mincle on microglia or inhibition of their downstream signaling may improve the prognosis of ischemic stroke.
Keywords: Ischemic stroke; Macrophages; Microglia; Mincle; Neutrophils; Properdin.
© 2023. The Author(s).
Conflict of interest statement
All authors have declared no competing interests.
Figures

Similar articles
-
Microglia LILRB4 upregulation reduces brain damage after acute ischemic stroke by limiting CD8+ T cell recruitment.J Neuroinflammation. 2024 Aug 31;21(1):214. doi: 10.1186/s12974-024-03206-4. J Neuroinflammation. 2024. PMID: 39217343 Free PMC article.
-
Receptor-interacting protein kinase 2 (RIPK2) profoundly contributes to post-stroke neuroinflammation and behavioral deficits with microglia as unique perpetrators.J Neuroinflammation. 2023 Sep 30;20(1):221. doi: 10.1186/s12974-023-02907-6. J Neuroinflammation. 2023. PMID: 37777791 Free PMC article.
-
Circular RNA PTP4A2 regulates microglial polarization through STAT3 to promote neuroinflammation in ischemic stroke.CNS Neurosci Ther. 2024 Apr;30(4):e14512. doi: 10.1111/cns.14512. Epub 2023 Oct 23. CNS Neurosci Ther. 2024. PMID: 37869777 Free PMC article.
-
Downregulation of Nogo-B ameliorates cerebral ischemia/reperfusion injury in mice through regulating microglia polarization via TLR4/NF-kappaB pathway.Neurochem Int. 2023 Jul;167:105553. doi: 10.1016/j.neuint.2023.105553. Epub 2023 May 23. Neurochem Int. 2023. PMID: 37230196 Review.
-
Flavonoids and ischemic stroke-induced neuroinflammation: Focus on the glial cells.Biomed Pharmacother. 2024 Jan;170:115847. doi: 10.1016/j.biopha.2023.115847. Epub 2023 Nov 27. Biomed Pharmacother. 2024. PMID: 38016362 Review.
Cited by
-
The Interplay between Ferroptosis and Neuroinflammation in Central Neurological Disorders.Antioxidants (Basel). 2024 Mar 26;13(4):395. doi: 10.3390/antiox13040395. Antioxidants (Basel). 2024. PMID: 38671843 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

