Urinary biomarkers for amyotrophic lateral sclerosis: candidates, opportunities and considerations
- PMID: 37946793
- PMCID: PMC10631861
- DOI: 10.1093/braincomms/fcad287
Urinary biomarkers for amyotrophic lateral sclerosis: candidates, opportunities and considerations
Erratum in
-
Correction to: Urinary biomarkers for amyotrophic lateral sclerosis: candidates, opportunities and considerations.Brain Commun. 2023 Dec 11;5(6):fcad334. doi: 10.1093/braincomms/fcad334. eCollection 2023. Brain Commun. 2023. PMID: 38090276 Free PMC article.
Abstract
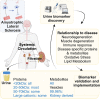
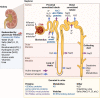
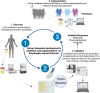
Amyotrophic lateral sclerosis is a relentless neurodegenerative disease that is mostly fatal within 3-5 years and is diagnosed on evidence of progressive upper and lower motor neuron degeneration. Around 15% of those with amyotrophic lateral sclerosis also have frontotemporal degeneration, and gene mutations account for ∼10%. Amyotrophic lateral sclerosis is a variable heterogeneous disease, and it is becoming increasingly clear that numerous different disease processes culminate in the final degeneration of motor neurons. There is a profound need to clearly articulate and measure pathological process that occurs. Such information is needed to tailor treatments to individuals with amyotrophic lateral sclerosis according to an individual's pathological fingerprint. For new candidate therapies, there is also a need for methods to select patients according to expected treatment outcomes and measure the success, or not, of treatments. Biomarkers are essential tools to fulfil these needs, and urine is a rich source for candidate biofluid biomarkers. This review will describe promising candidate urinary biomarkers of amyotrophic lateral sclerosis and other possible urinary candidates in future areas of investigation as well as the limitations of urinary biomarkers.
Keywords: ALS; biomarker; metabolites; proteins; urine.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures
Comment in
-
Correction to: Urinary biomarkers for amyotrophic lateral sclerosis: candidates, opportunities and considerations.Brain Commun. 2023 Dec 11;5(6):fcad334. doi: 10.1093/braincomms/fcad334. eCollection 2023. Brain Commun. 2023. PMID: 38090276 Free PMC article.
Similar articles
-
A Review of Biomarkers of Amyotrophic Lateral Sclerosis: A Pathophysiologic Approach.Int J Mol Sci. 2024 Oct 10;25(20):10900. doi: 10.3390/ijms252010900. Int J Mol Sci. 2024. PMID: 39456682 Free PMC article. Review.
-
Biofluid Biomarkers in the Prognosis of Amyotrophic Lateral Sclerosis: Recent Developments and Therapeutic Applications.Cells. 2023 Apr 18;12(8):1180. doi: 10.3390/cells12081180. Cells. 2023. PMID: 37190090 Free PMC article. Review.
-
Skeletal muscle as a molecular and cellular biomarker of disease progression in amyotrophic lateral sclerosis: a narrative review.Neural Regen Res. 2024 Apr;19(4):747-753. doi: 10.4103/1673-5374.382226. Neural Regen Res. 2024. PMID: 37843208 Free PMC article. Review.
-
Motor neuron TDP-43 proteinopathy in progressive supranuclear palsy and corticobasal degeneration.Brain. 2022 Aug 27;145(8):2769-2784. doi: 10.1093/brain/awac091. Brain. 2022. PMID: 35274674
-
Current application of neurofilaments in amyotrophic lateral sclerosis and future perspectives.Neural Regen Res. 2021 Oct;16(10):1985-1991. doi: 10.4103/1673-5374.308072. Neural Regen Res. 2021. PMID: 33642372 Free PMC article. Review.
Cited by
-
Brain-body mechanisms contribute to sexual dimorphism in amyotrophic lateral sclerosis.Nat Rev Neurol. 2024 Aug;20(8):475-494. doi: 10.1038/s41582-024-00991-7. Epub 2024 Jul 4. Nat Rev Neurol. 2024. PMID: 38965379 Review.
-
Prognostic Clinical and Biological Markers for Amyotrophic Lateral Sclerosis Disease Progression: Validation and Implications for Clinical Trial Design and Analysis.medRxiv [Preprint]. 2024 Aug 13:2024.08.12.24311876. doi: 10.1101/2024.08.12.24311876. medRxiv. 2024. Update in: EBioMedicine. 2024 Oct;108:105323. doi: 10.1016/j.ebiom.2024.105323 PMID: 39185513 Free PMC article. Updated. Preprint.
-
Biomarkers for Managing Neurodegenerative Diseases.Biomolecules. 2024 Mar 26;14(4):398. doi: 10.3390/biom14040398. Biomolecules. 2024. PMID: 38672416 Free PMC article. Review.
References
-
- Genge A, Chio A. The future of ALS diagnosis and staging: Where do we go from here? Amyotroph Lateral Scler Frontotemporal Degener. 2023;24(3–4):165–174. - PubMed
-
- Atkinson A, Colburn W, DeGruttola V, et al. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework*. Clin Pharmacol Ther. 2001;69(3):89–95. - PubMed
-
- BEST F-NBWG. FDA-NIH Biomarker Working Group . BEST (Biomarkers, EndpointS, and other tools) resource [internet]. In: 2016 FaDAU, ed. last Updated November 2021 ed. Silver Spring, Bethesda (MD). Co-published by National Institutes of Health (US). 2021:45.
-
- Rooney J, Burke T, Vajda A, Heverin M, Hardiman O. What does the ALSFRS-R really measure? A longitudinal and survival analysis of functional dimension subscores in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2017;88(5):381–385. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
