M2 macrophage-derived cathepsin S promotes peripheral nerve regeneration via fibroblast-Schwann cell-signaling relay
- PMID: 37946211
- PMCID: PMC10636844
- DOI: 10.1186/s12974-023-02943-2
M2 macrophage-derived cathepsin S promotes peripheral nerve regeneration via fibroblast-Schwann cell-signaling relay
Abstract
Background: Although peripheral nerves have an intrinsic self-repair capacity following damage, functional recovery is limited in patients. It is a well-established fact that macrophages accumulate at the site of injury. Numerous studies indicate that the phenotypic shift from M1 macrophage to M2 macrophage plays a crucial role in the process of axon regeneration. This polarity change is observed exclusively in peripheral macrophages but not in microglia and CNS macrophages. However, the molecular basis of axonal regeneration by M2 macrophage is not yet fully understood. Herein, we aimed to identify the M2 macrophage-derived axon regeneration factor.
Methods: We established a peripheral nerve injury model by transection of the inferior alveolar nerve (IANX) in Sprague-Dawley rats. Transcriptome analysis was performed on the injured nerve. Recovery from sensory deficits in the mandibular region and histological reconnection of IAN after IANX were assessed in rats with macrophage depletion by clodronate. We investigated the effects of adoptive transfer of M2 macrophages or M2-derived cathepsin S (CTSS) on the sensory deficit. CTSS initiating signaling was explored by western blot analysis in IANX rats and immunohistochemistry in co-culture of primary fibroblasts and Schwann cells (SCs).
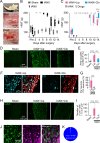
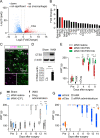
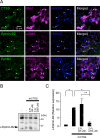
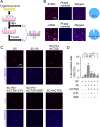
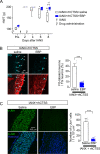
Results: Transcriptome analysis revealed that CTSS, a macrophage-selective lysosomal protease, was upregulated in the IAN after its injury. Spontaneous but partial recovery from a sensory deficit in the mandibular region after IANX was abrogated by macrophage ablation at the injured site. In addition, a robust induction of c-Jun, a marker of the repair-supportive phenotype of SCs, after IANX was abolished by macrophage ablation. As in transcriptome analysis, CTSS was upregulated at the injured IAN than in the intact IAN. Endogenous recovery from hypoesthesia was facilitated by supplementation of CTSS but delayed by pharmacological inhibition or genetic silencing of CTSS at the injured site. Adoptive transfer of M2-polarized macrophages at this site facilitated sensory recovery dependent on CTSS in macrophages. Post-IANX, CTSS caused the cleavage of Ephrin-B2 in fibroblasts, which, in turn, bound EphB2 in SCs. CTSS-induced Ephrin-B2 cleavage was also observed in human sensory nerves. Inhibition of CTSS-induced Ephrin-B2 signaling suppressed c-Jun induction in SCs and sensory recovery.
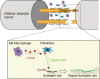
Conclusions: These results suggest that M2 macrophage-derived CTSS contributes to axon regeneration by activating SCs via Ephrin-B2 shedding from fibroblasts.
Keywords: Axon regeneration; Cathepsin S; Ephrin-B2; Gene expression; Macrophage; Peripheral nerve injury; Schwann cells.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Down-regulation miR-146a-5p in Schwann cell-derived exosomes induced macrophage M1 polarization by impairing the inhibition on TRAF6/NF-κB pathway after peripheral nerve injury.Exp Neurol. 2023 Apr;362:114295. doi: 10.1016/j.expneurol.2022.114295. Epub 2022 Dec 6. Exp Neurol. 2023. PMID: 36493861
-
Rapamycin Accelerates Axon Regeneration Through Schwann Cell-mediated Autophagy Following Inferior Alveolar Nerve Transection in Rats.Neuroscience. 2021 Aug 1;468:43-52. doi: 10.1016/j.neuroscience.2021.05.033. Epub 2021 Jun 5. Neuroscience. 2021. PMID: 34102263
-
Postinjury Induction of Activated ErbB2 Selectively Hyperactivates Denervated Schwann Cells and Promotes Robust Dorsal Root Axon Regeneration.J Neurosci. 2017 Nov 8;37(45):10955-10970. doi: 10.1523/JNEUROSCI.0903-17.2017. Epub 2017 Oct 5. J Neurosci. 2017. PMID: 28982707 Free PMC article.
-
Applications of Proteomics to Nerve Regeneration Research.In: Alzate O, editor. Neuroproteomics. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 15. In: Alzate O, editor. Neuroproteomics. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 15. PMID: 21882439 Free Books & Documents. Review.
-
Peripheral Nerve Regeneration and Muscle Reinnervation.Int J Mol Sci. 2020 Nov 17;21(22):8652. doi: 10.3390/ijms21228652. Int J Mol Sci. 2020. PMID: 33212795 Free PMC article. Review.
Cited by
-
Adoptive transfer of immunomodulatory macrophages reduces the pro-inflammatory microenvironment and increases bone formation on titanium implants.Acta Biomater. 2024 Oct 15;188:432-445. doi: 10.1016/j.actbio.2024.09.011. Epub 2024 Sep 16. Acta Biomater. 2024. PMID: 39293568
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

