Synaptic proteasome is inhibited in Alzheimer's disease models and associates with memory impairment in mice
- PMID: 37935829
- PMCID: PMC10630330
- DOI: 10.1038/s42003-023-05511-9
Synaptic proteasome is inhibited in Alzheimer's disease models and associates with memory impairment in mice
Abstract
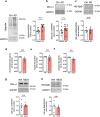
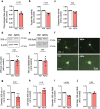
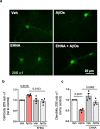
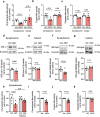
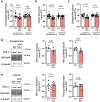
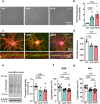
The proteasome plays key roles in synaptic plasticity and memory by regulating protein turnover, quality control, and elimination of oxidized/misfolded proteins. Here, we investigate proteasome function and localization at synapses in Alzheimer's disease (AD) post-mortem brain tissue and in experimental models. We found a marked increase in ubiquitinylated proteins in post-mortem AD hippocampi compared to controls. Using several experimental models, we show that amyloid-β oligomers (AβOs) inhibit synaptic proteasome activity and trigger a reduction in synaptic proteasome content. We further show proteasome inhibition specifically in hippocampal synaptic fractions derived from APPswePS1ΔE9 mice. Reduced synaptic proteasome activity instigated by AβOs is corrected by treatment with rolipram, a phosphodiesterase-4 inhibitor, in mice. Results further show that dynein inhibition blocks AβO-induced reduction in dendritic proteasome content in hippocampal neurons. Finally, proteasome inhibition induces AD-like pathological features, including reactive oxygen species and dendritic spine loss in hippocampal neurons, inhibition of hippocampal mRNA translation, and memory impairment in mice. Results suggest that proteasome inhibition may contribute to synaptic and memory deficits in AD.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Astrocyte Transforming Growth Factor Beta 1 Protects Synapses against Aβ Oligomers in Alzheimer's Disease Model.J Neurosci. 2017 Jul 12;37(28):6797-6809. doi: 10.1523/JNEUROSCI.3351-16.2017. Epub 2017 Jun 12. J Neurosci. 2017. PMID: 28607171 Free PMC article.
-
Interaction of amyloid-β (Aβ) oligomers with neurexin 2α and neuroligin 1 mediates synapse damage and memory loss in mice.J Biol Chem. 2017 May 5;292(18):7327-7337. doi: 10.1074/jbc.M116.761189. Epub 2017 Mar 10. J Biol Chem. 2017. PMID: 28283575 Free PMC article.
-
Xingnaojing ameliorates synaptic plasticity and memory deficits in an Aβ1-42 induced mouse model of Alzheimer's disease.J Pharmacol Sci. 2020 Aug;143(4):245-254. doi: 10.1016/j.jphs.2020.05.002. Epub 2020 May 14. J Pharmacol Sci. 2020. PMID: 32482409
-
Amyloid-β oligomers synaptotoxicity: The emerging role of EphA4/c-Abl signaling in Alzheimer's disease.Biochim Biophys Acta Mol Basis Dis. 2018 Apr;1864(4 Pt A):1148-1159. doi: 10.1016/j.bbadis.2018.01.023. Epub 2018 Jan 31. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 29378302 Review.
-
Amyloid β oligomers in Alzheimer's disease pathogenesis, treatment, and diagnosis.Acta Neuropathol. 2015 Feb;129(2):183-206. doi: 10.1007/s00401-015-1386-3. Epub 2015 Jan 22. Acta Neuropathol. 2015. PMID: 25604547 Free PMC article. Review.
Cited by
-
Proteasome hyperactivation rewires the proteome enhancing stress resistance, proteostasis, lipid metabolism and ERAD in C. elegans.bioRxiv [Preprint]. 2024 Apr 6:2024.04.04.588128. doi: 10.1101/2024.04.04.588128. bioRxiv. 2024. PMID: 38617285 Free PMC article. Preprint.
-
Alzheimer's disease risk gene CD2AP is a dose-sensitive determinant of synaptic structure and plasticity.Hum Mol Genet. 2024 Oct 7;33(20):1815-1832. doi: 10.1093/hmg/ddae115. Hum Mol Genet. 2024. PMID: 39146503 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

