Advancing bovine in vitro fertilization through 3D printing: the effect of the 3D printed materials
- PMID: 37929185
- PMCID: PMC10621798
- DOI: 10.3389/fbioe.2023.1260886
Advancing bovine in vitro fertilization through 3D printing: the effect of the 3D printed materials
Abstract
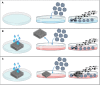
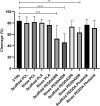
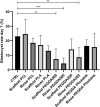
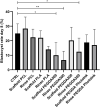
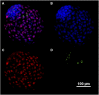
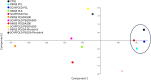
Nowadays there is an increasing demand for assisted reproductive technologies due to the growth of infertility problems. Naturally, fertilization occurs in the oviduct, where the oviductal epithelial cells (OECs) secrete many molecules that affect the embryo's metabolism and protect it from oxidative stress. When the OECs are grown in 3D culture systems, they maintain a great part of their functional characteristics, making them an excellent model for in vitro fertilization (IVF) studies. In this work, we aimed to evaluate the suitability of different 3D-printing processes in conjunction with the corresponding set of commercially available biomaterials: extrusion-based processing using polylactic acid (PLA) and polycaprolactone (PCL) and stereolithography or digital-light processing using polyethylene-glycol-diacrylate (PEGDA) with different stiffness (PEGDA500, PEGDA200, PEGDA PhotoInk). All the 3D-printed scaffolds were used to support IVF process in a bovine embryo assay. Following fertilization, embryo development and quality were assessed in terms of cleavage, blastocyst rate at days 7 and 8, total cell number (TCN), inner cell mass/trophectoderm ratio (ICN/TE), and apoptotic cell ratio (ACR). We found a detrimental effect on cleavage and blastocyst rates when the IVF was performed on any medium conditioned by most of the materials available for digital-light processing (PEGDA200, PEGDA500). The observed negative effect could be possibly due to some leaked compound used to print and stabilize the scaffolds, which was not so evident however with PEGDA PhotoInk. On the other hand, all the extrusion-based processable materials did not cause any detrimental effect on cleavage or blastocyst rates. The principal component analysis reveals that embryos produced in presence of 3D-printed scaffolds produced via extrusion exhibit the highest similarity with the control embryos considering cleavage, blastocyst rates, TCN, ICN/TE and ACR per embryo. Conversely, all the photo-cross linkable materials or medium conditioned by PLA, lead to the highest dissimilarities. Since the use of PCL scaffolds, as well as its conditioned medium, bring to embryos that are more similar to the control group. Our results suggest that extrusion-based 3D printing of PCL could be the best option to be used for new IVF devices, possibly including the support of OECs, to enhance bovine embryo development.
Keywords: IVF; PCL; PEGDA; PLA; biomaterials; bovine; embryo culture.
Copyright © 2023 Belda-Perez, Heras, Cimini, Romero-Aguirregomezcorta, Valbonetti, Colosimo, Colosimo, Santoni, Barboni, Bernabò and Coy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Antioxidants improve IVF outcome and subsequent embryo development in the mouse.Hum Reprod. 2017 Dec 1;32(12):2404-2413. doi: 10.1093/humrep/dex330. Hum Reprod. 2017. PMID: 29136144
-
Corticotrophin-releasing hormone and corticosterone impair development of preimplantation embryos by inducing oviductal cell apoptosis via activating the Fas system: an in vitro study.Hum Reprod. 2017 Aug 1;32(8):1583-1597. doi: 10.1093/humrep/dex217. Hum Reprod. 2017. PMID: 28591825
-
Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering.Mater Sci Eng C Mater Biol Appl. 2019 Nov;104:109960. doi: 10.1016/j.msec.2019.109960. Epub 2019 Jul 6. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 31500051
-
Guidelines for the number of embryos to transfer following in vitro fertilization No. 182, September 2006.Int J Gynaecol Obstet. 2008 Aug;102(2):203-16. doi: 10.1016/j.ijgo.2008.01.007. Int J Gynaecol Obstet. 2008. PMID: 18773532 Review.
-
Is 3D Printing Promising for Osteochondral Tissue Regeneration?ACS Appl Bio Mater. 2023 Apr 17;6(4):1431-1444. doi: 10.1021/acsabm.3c00093. Epub 2023 Mar 21. ACS Appl Bio Mater. 2023. PMID: 36943415 Free PMC article. Review.
Cited by
-
Exploring swine oviduct anatomy through micro-computed tomography: a 3D modeling perspective.Front Vet Sci. 2024 Sep 3;11:1456524. doi: 10.3389/fvets.2024.1456524. eCollection 2024. Front Vet Sci. 2024. PMID: 39290503 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

