Novel PD-L1- and collagen-expressing patient-derived cell line of undifferentiated pleomorphic sarcoma (JBT19) as a model for cancer immunotherapy
- PMID: 37925511
- PMCID: PMC10625569
- DOI: 10.1038/s41598-023-46305-7
Novel PD-L1- and collagen-expressing patient-derived cell line of undifferentiated pleomorphic sarcoma (JBT19) as a model for cancer immunotherapy
Abstract
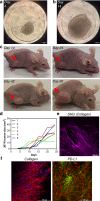
Soft tissue sarcomas are aggressive mesenchymal-origin malignancies. Undifferentiated pleomorphic sarcoma (UPS) belongs to the aggressive, high-grade, and least characterized sarcoma subtype, affecting multiple tissues and metastasizing to many organs. The treatment of localized UPS includes surgery in combination with radiation therapy. Metastatic forms are treated with chemotherapy. Immunotherapy is a promising treatment modality for many cancers. However, the development of immunotherapy for UPS is limited due to its heterogeneity, antigenic landscape variation, lower infiltration with immune cells, and a limited number of established patient-derived UPS cell lines for preclinical research. In this study, we established and characterized a novel patient-derived UPS cell line, JBT19. The JBT19 cells express PD-L1 and collagen, a ligand of the immune checkpoint molecule LAIR-1. JBT19 cells can form spheroids in vitro and solid tumors in immunodeficient nude mice. We found JBT19 cells induce expansion of JBT19-reactive autologous and allogeneic NK, T, and NKT-like cells, and the reactivity of the expanded cells was associated with cytotoxic impact on JBT19 cells. The PD-1 and LAIR-1 ligand-expressing JBT19 cells show ex vivo immunogenicity and effective in vivo xenoengraftment properties that can offer a unique resource in the preclinical research developing novel immunotherapeutic interventions in the treatment of UPS.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








Similar articles
-
T-cell infiltration and clonality correlate with programmed cell death protein 1 and programmed death-ligand 1 expression in patients with soft tissue sarcomas.Cancer. 2017 Sep 1;123(17):3291-3304. doi: 10.1002/cncr.30726. Epub 2017 May 2. Cancer. 2017. PMID: 28463396 Free PMC article.
-
Establishment and characterization of NCC-UPS3-C1: a novel patient-derived cell line of undifferentiated pleomorphic sarcoma.Hum Cell. 2022 Jan;35(1):384-391. doi: 10.1007/s13577-021-00633-w. Epub 2021 Oct 19. Hum Cell. 2022. PMID: 34665443
-
Evaluation of PD-L1 Expression in Undifferentiated Pleomorphic Sarcomas, Liposarcomas and Chondrosarcomas.Biomolecules. 2022 Feb 11;12(2):292. doi: 10.3390/biom12020292. Biomolecules. 2022. PMID: 35204793 Free PMC article.
-
A League of Its Own? Established and Emerging Therapies in Undifferentiated Pleomorphic Sarcoma.Curr Treat Options Oncol. 2023 Mar;24(3):212-228. doi: 10.1007/s11864-023-01054-7. Epub 2023 Feb 2. Curr Treat Options Oncol. 2023. PMID: 36729198 Review.
-
PD-L1 expression in sarcomas: An immunohistochemical study and review of the literature.Ann Diagn Pathol. 2021 Dec;55:151823. doi: 10.1016/j.anndiagpath.2021.151823. Epub 2021 Sep 4. Ann Diagn Pathol. 2021. PMID: 34656856 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

