Mucosal immune alterations at the early onset of tissue destruction in chronic obstructive pulmonary disease
- PMID: 37915582
- PMCID: PMC10616299
- DOI: 10.3389/fimmu.2023.1275845
Mucosal immune alterations at the early onset of tissue destruction in chronic obstructive pulmonary disease
Abstract
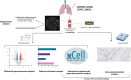
Rationale: COPD is characterized by chronic airway inflammation, small airways changes, with disappearance and obstruction, and also distal/alveolar destruction (emphysema). The chronology by which these three features evolve with altered mucosal immunity remains elusive. This study assessed the mucosal immune defense in human control and end-stage COPD lungs, by detailed microCT and RNA transcriptomic analysis of diversely affected zones.
Methods: In 11 control (non-used donors) and 11 COPD (end-stage) explant frozen lungs, 4 cylinders/cores were processed per lung for microCT and tissue transcriptomics. MicroCT was used to quantify tissue percentage and alveolar surface density to classify the COPD cores in mild, moderate and severe alveolar destruction groups, as well as to quantify terminal bronchioles in each group. Transcriptomics of each core assessed fold changes in innate and adaptive cells and pathway enrichment score between control and COPD cores. Immunostainings of immune cells were performed for validation.
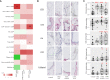
Results: In mildly affected zones, decreased defensins and increased mucus production were observed, along CD8+ T cell accumulation and activation of the IgA pathway. In more severely affected zones, CD68+ myeloid antigen-presenting cells, CD4+ T cells and B cells, as well as MHCII and IgA pathway genes were upregulated. In contrast, terminal bronchioles were decreased in all COPD cores.
Conclusion: Spatial investigation of end-stage COPD lungs show that mucosal defense dysregulation with decreased defensins and increased mucus and IgA responses, start concomitantly with CD8+ T-cell accumulation in mild emphysema zones, where terminal bronchioles are already decreased. In contrast, adaptive Th and B cell activation is observed in areas with more advanced tissue destruction. This study suggests that in COPD innate immune alterations occur early in the tissue destruction process, which affects both the alveoli and the terminal bronchioles, before the onset of an adaptive immune response.
Keywords: airway inflammation; chronic obstructive pulmonary disease; emphysema severity; lung mucosal immunity; lung tissue destruction.
Copyright © 2023 de Fays, Geudens, Gyselinck, Kerckhof, Vermaut, Goos, Vermant, Beeckmans, Kaes, Van Slambrouck, Mohamady, Willems, Aversa, Cortesi, Hooft, Aerts, Aelbrecht, Everaerts, McDonough, De Sadeleer, Gohy, Ambroise, Janssens, Ceulemans, Van Raemdonck, Vos, Hackett, Hogg, Kaminski, Gayan-Ramirez, Pilette and Vanaudenaerde.
Conflict of interest statement
IG reports travel support from AstraZeneca, not related to the content of this work. MV reports travel support from Sanofi, not related to the content of this manuscript. SE reports consulting fees from GSK, lecture honoraria from GSK, Boehringer Ingelheim, Chiesi and AstraZeneca, travel support from GSK and Sanofi, not related to the content of this manuscript. WJ reports grants and lecture honoraria from AstraZeneca and Chiesi, consulting fees from AstraZeneca, Griffols, GSK and Chiesi and travel support from Chiesi and AstraZeneca; has a leadership or fiduciary role in Board of VRGT and Board of ArtiQ, and is a co-founder and shareholder of ArtiQ NV. NK served as consultant for Biogen Idec, Boehringer Ingelheim, Third Rock, Pliant, Samumed, NuMedii, Theravance, LifeMax, Three Lake Partners, Astra Zeneca, RohBar, Veracyte, Augmanity, CSL Behring, Thyron, BMS, Biotech, Gilead, Galapagos, Chiesi, Arrowhead, Sofinnova and GSK, reports equity in Pliant and Thyron, and has a patent in new therapies for IPF, new therapies for ARDS and new biomarkers for IPF, licensed to Biotech. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Small-airway obstruction and emphysema in chronic obstructive pulmonary disease.N Engl J Med. 2011 Oct 27;365(17):1567-75. doi: 10.1056/NEJMoa1106955. N Engl J Med. 2011. PMID: 22029978 Free PMC article.
-
Small Airway Disease in Pre-Chronic Obstructive Pulmonary Disease with Emphysema: A Cross-Sectional Study.Am J Respir Crit Care Med. 2024 Mar 15;209(6):683-692. doi: 10.1164/rccm.202301-0132OC. Am J Respir Crit Care Med. 2024. PMID: 38055196
-
Pathological Comparisons of Paraseptal and Centrilobular Emphysema in Chronic Obstructive Pulmonary Disease.Am J Respir Crit Care Med. 2020 Sep 15;202(6):803-811. doi: 10.1164/rccm.201912-2327OC. Am J Respir Crit Care Med. 2020. PMID: 32485111
-
Inflammatory cells and chronic obstructive pulmonary disease.Curr Drug Targets Inflamm Allergy. 2005 Dec;4(6):607-18. doi: 10.2174/156801005774912824. Curr Drug Targets Inflamm Allergy. 2005. PMID: 17305517 Review.
-
Lung structure and function in COPD.Int J Tuberc Lung Dis. 2008 May;12(5):467-79. Int J Tuberc Lung Dis. 2008. PMID: 18419881 Review.
Cited by
-
The Current Molecular and Cellular Landscape of Chronic Obstructive Pulmonary Disease (COPD): A Review of Therapies and Efforts towards Personalized Treatment.Proteomes. 2024 Aug 16;12(3):23. doi: 10.3390/proteomes12030023. Proteomes. 2024. PMID: 39189263 Free PMC article. Review.
-
Pulmonary and Systemic Immune Profiles Following Lung Volume Reduction Surgery and Allogeneic Mesenchymal Stromal Cell Treatment in Emphysema.Cells. 2024 Sep 30;13(19):1636. doi: 10.3390/cells13191636. Cells. 2024. PMID: 39404398 Free PMC article.
-
Dissecting causal relationships between immune cells, plasma metabolites, and COPD: a mediating Mendelian randomization study.Front Immunol. 2024 May 28;15:1406234. doi: 10.3389/fimmu.2024.1406234. eCollection 2024. Front Immunol. 2024. PMID: 38868780 Free PMC article.
References
-
- Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. . Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (2020) 396(10258):1204–22. doi: 10.1016/S0140-6736(20)30925-9 - DOI - PMC - PubMed
-
- Organization WH . The top 10 causes of death 2020. Available at: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

