DNA damage response(DDR): a link between cellular senescence and human cytomegalovirus
- PMID: 37915066
- PMCID: PMC10621139
- DOI: 10.1186/s12985-023-02203-y
DNA damage response(DDR): a link between cellular senescence and human cytomegalovirus
Abstract
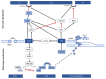
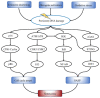
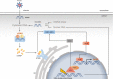
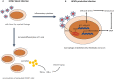
The DNA damage response (DDR) is a signaling cascade that is triggered by DNA damage, involving the halting of cell cycle progression and repair. It is a key event leading to senescence, which is characterized by irreversible cell cycle arrest and the senescence-associated secretory phenotype (SASP) that includes the expression of inflammatory cytokines. Human cytomegalovirus (HCMV) is a ubiquitous pathogen that plays an important role in the senescence process. It has been established that DDR is necessary for HCMV to replicate effectively. This paper reviews the relationship between DDR, cellular senescence, and HCMV, providing new sights for virus-induced senescence (VIS).
Keywords: Cell cycle; Cellular senescence; DNA damage; Human cytomegalovirus; Senescence-associated secretory phenotype.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
MLL1 is essential for the senescence-associated secretory phenotype.Genes Dev. 2016 Feb 1;30(3):321-36. doi: 10.1101/gad.271882.115. Genes Dev. 2016. PMID: 26833731 Free PMC article.
-
Quantifying Senescence-Associated Phenotypes in Primary Multipotent Mesenchymal Stromal Cell Cultures.Methods Mol Biol. 2019;2045:93-105. doi: 10.1007/7651_2019_217. Methods Mol Biol. 2019. PMID: 31020633
-
High-LET-Radiation-Induced Persistent DNA Damage Response Signaling and Gastrointestinal Cancer Development.Curr Oncol. 2023 Jun 7;30(6):5497-5514. doi: 10.3390/curroncol30060416. Curr Oncol. 2023. PMID: 37366899 Free PMC article. Review.
-
Alteration in the chromatin landscape during the DNA damage response: Continuous rotation of the gear driving cellular senescence and aging.DNA Repair (Amst). 2023 Nov;131:103572. doi: 10.1016/j.dnarep.2023.103572. Epub 2023 Sep 17. DNA Repair (Amst). 2023. PMID: 37742405 Review.
-
Partial sleep deprivation activates the DNA damage response (DDR) and the senescence-associated secretory phenotype (SASP) in aged adult humans.Brain Behav Immun. 2016 Jan;51:223-229. doi: 10.1016/j.bbi.2015.08.024. Epub 2015 Aug 31. Brain Behav Immun. 2016. PMID: 26336034 Free PMC article.
Cited by
-
Recent advancements in cGAS-STING activation, tumor immune evasion, and therapeutic implications.Med Oncol. 2024 Oct 18;41(11):291. doi: 10.1007/s12032-024-02539-7. Med Oncol. 2024. PMID: 39419913 Review.
-
YIPF2 regulates genome integrity.Cell Biosci. 2024 Sep 5;14(1):114. doi: 10.1186/s13578-024-01300-x. Cell Biosci. 2024. PMID: 39238039 Free PMC article.
-
The Molecular Mechanisms in Senescent Cells Induced by Natural Aging and Ionizing Radiation.Cells. 2024 Mar 21;13(6):550. doi: 10.3390/cells13060550. Cells. 2024. PMID: 38534394 Free PMC article. Review.
-
Crosstalk between the DNA damage response and cellular senescence drives aging and age-related diseases.Semin Immunopathol. 2024 Aug 2;46(3-4):10. doi: 10.1007/s00281-024-01016-7. Semin Immunopathol. 2024. PMID: 39095660 Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

