Replisome loading reduces chromatin motion independent of DNA synthesis
- PMID: 37906089
- PMCID: PMC10617993
- DOI: 10.7554/eLife.87572
Replisome loading reduces chromatin motion independent of DNA synthesis
Abstract
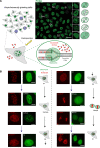
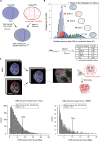
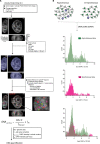
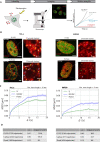
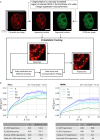
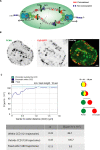
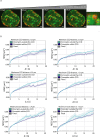
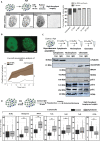
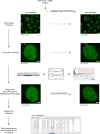
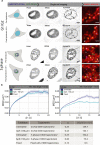
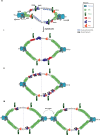
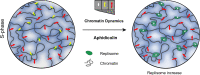
Chromatin has been shown to undergo diffusional motion, which is affected during gene transcription by RNA polymerase activity. However, the relationship between chromatin mobility and other genomic processes remains unclear. Hence, we set out to label the DNA directly in a sequence unbiased manner and followed labeled chromatin dynamics in interphase human cells expressing GFP-tagged proliferating cell nuclear antigen (PCNA), a cell cycle marker and core component of the DNA replication machinery. We detected decreased chromatin mobility during the S-phase compared to G1 and G2 phases in tumor as well as normal diploid cells using automated particle tracking. To gain insight into the dynamical organization of the genome during DNA replication, we determined labeled chromatin domain sizes and analyzed their motion in replicating cells. By correlating chromatin mobility proximal to the active sites of DNA synthesis, we showed that chromatin motion was locally constrained at the sites of DNA replication. Furthermore, inhibiting DNA synthesis led to increased loading of DNA polymerases. This was accompanied by accumulation of the single-stranded DNA binding protein on the chromatin and activation of DNA helicases further restricting local chromatin motion. We, therefore, propose that it is the loading of replisomes but not their catalytic activity that reduces the dynamics of replicating chromatin segments in the S-phase as well as their accessibility and probability of interactions with other genomic regions.
Keywords: DNA labeling; DNA replication; aphidicolin; cell biology; cell cycle; chromatin tracking; diffusion; human.
© 2023, Pabba, Ritter, Chagin et al.
Conflict of interest statement
MP, CR, VC, JM, KC, JS, DL, KK, PP, AS, HL, KR, MC No competing interests declared
Figures







Update of
- doi: 10.1101/2023.03.07.531331
- doi: 10.7554/eLife.87572.1
- doi: 10.7554/eLife.87572.2
Similar articles
-
PCNA is recruited to irradiated chromatin in late S-phase and is most pronounced in G2 phase of the cell cycle.Protoplasma. 2017 Sep;254(5):2035-2043. doi: 10.1007/s00709-017-1076-1. Epub 2017 Jan 20. Protoplasma. 2017. PMID: 28168519
-
Human replication proteins hCdc21, hCdc46 and P1Mcm3 bind chromatin uniformly before S-phase and are displaced locally during DNA replication.J Cell Sci. 1996 Feb;109 ( Pt 2):309-18. doi: 10.1242/jcs.109.2.309. J Cell Sci. 1996. PMID: 8838654
-
Dynamic changes in subnuclear NP95 location during the cell cycle and its spatial relationship with DNA replication foci.Exp Cell Res. 2001 Feb 15;263(2):202-8. doi: 10.1006/excr.2000.5115. Exp Cell Res. 2001. PMID: 11161719
-
Chromosome Duplication in Saccharomyces cerevisiae.Genetics. 2016 Jul;203(3):1027-67. doi: 10.1534/genetics.115.186452. Genetics. 2016. PMID: 27384026 Free PMC article. Review.
-
Control of Genome Integrity by RFC Complexes; Conductors of PCNA Loading onto and Unloading from Chromatin during DNA Replication.Genes (Basel). 2017 Jan 26;8(2):52. doi: 10.3390/genes8020052. Genes (Basel). 2017. PMID: 28134787 Free PMC article. Review.
Cited by
-
Timeless-Tipin interactions with MCM and RPA mediate DNA replication stress response.Front Cell Dev Biol. 2024 Feb 29;12:1346534. doi: 10.3389/fcell.2024.1346534. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38487270 Free PMC article.
-
DNA choreography: correlating mobility and organization of DNA across different resolutions from loops to chromosomes.Histochem Cell Biol. 2024 Jul;162(1-2):109-131. doi: 10.1007/s00418-024-02285-x. Epub 2024 May 17. Histochem Cell Biol. 2024. PMID: 38758428 Free PMC article.
References
-
- Baddeley D, Chagin VO, Schermelleh L, Martin S, Pombo A, Carlton PM, Gahl A, Domaing P, Birk U, Leonhardt H, Cremer C, Cardoso MC. Measurement of replication structures at the nanometer scale using super-resolution light microscopy. Nucleic Acids Research. 2010;38:e8. doi: 10.1093/nar/gkp901. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

