This is a preprint.
Inflammatory stress-mediated chromatin changes underlie dysfunction in endothelial cells
- PMID: 37905100
- PMCID: PMC10614786
- DOI: 10.1101/2023.10.11.561959
Inflammatory stress-mediated chromatin changes underlie dysfunction in endothelial cells
Abstract
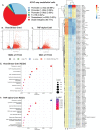
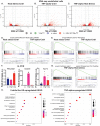
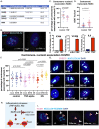
Inflammatory stresses underlie endothelial dysfunction and contribute to the development of chronic cardiovascular disorders such as atherosclerosis and vascular fibrosis. The initial transcriptional response of endothelial cells to pro-inflammatory cytokines such as TNF-alpha is well established. However, very few studies uncover the effects of inflammatory stresses on chromatin architecture. We used integrative analysis of ATAC-seq and RNA-seq data to investigate chromatin alterations in human endothelial cells in response to TNF-alpha and febrile-range heat stress exposure. Multi-omics data analysis suggests a correlation between the transcription of stress-related genes and endothelial dysfunction drivers with chromatin regions exhibiting differential accessibility. Moreover, microscopy identified the dynamics in the nuclear organization, specifically, the changes in a subset of heterochromatic nucleoli-associated chromatin domains, the centromeres. Upon inflammatory stress exposure, the centromeres decreased association with nucleoli in a p38-dependent manner and increased the number of transcripts from pericentromeric regions. Overall, we provide two lines of evidence that suggest chromatin alterations in vascular endothelial cells during inflammatory stresses.
Keywords: TNF-alpha; centromeres; chromatin; cytokines; febrile; heat stress; heterochromatin; inflammation; stress; vascular endothelial cells.
Conflict of interest statement
DECLARATION OF INTEREST The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Role of Histone Variant H2A.J in Fine-Tuning Chromatin Organization for the Establishment of Ionizing Radiation-Induced Senescence.Cells. 2023 Mar 16;12(6):916. doi: 10.3390/cells12060916. Cells. 2023. PMID: 36980257 Free PMC article.
-
Characterization of the chromatin accessibility in an Alzheimer's disease (AD) mouse model.Alzheimers Res Ther. 2020 Mar 23;12(1):29. doi: 10.1186/s13195-020-00598-2. Alzheimers Res Ther. 2020. PMID: 32293531 Free PMC article.
-
Endothelial GATA4 controls liver fibrosis and regeneration by preventing a pathogenic switch in angiocrine signaling.J Hepatol. 2021 Feb;74(2):380-393. doi: 10.1016/j.jhep.2020.08.033. Epub 2020 Sep 9. J Hepatol. 2021. PMID: 32916216
-
Computational Integration of HSV-1 Multi-omics Data.Methods Mol Biol. 2023;2610:31-48. doi: 10.1007/978-1-0716-2895-9_3. Methods Mol Biol. 2023. PMID: 36534279 Review.
-
Pro-inflammatory cytokines mediating senescence of vascular endothelial cells in atherosclerosis.Fundam Clin Pharmacol. 2023 Oct;37(5):928-936. doi: 10.1111/fcp.12915. Epub 2023 May 25. Fundam Clin Pharmacol. 2023. PMID: 37154136 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
