AMPK activation induces immunogenic cell death in AML
- PMID: 37903311
- PMCID: PMC10733104
- DOI: 10.1182/bloodadvances.2022009444
AMPK activation induces immunogenic cell death in AML
Abstract
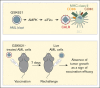
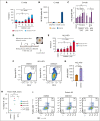
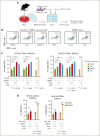
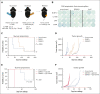
Survival of patients with acute myeloid leukemia (AML) can be improved by allogeneic hematopoietic stem cell transplantation (allo-HSCT) because of the antileukemic activity of T and natural killer cells from the donor. However, the use of allo-HSCT is limited by donor availability, recipient age, and potential severe side effects. Similarly, the efficacy of immunotherapies directing autologous T cells against tumor cells, including T-cell recruiting antibodies, chimeric antigen receptor T-cell therapy, and immune checkpoint inhibitors are limited in AML because of multiple mechanisms of leukemia immune escape. This has prompted a search for novel immunostimulatory approaches. Here, we show that activation of adenosine 5'-monophosphate-activated protein kinase (AMPK), a master regulator of cellular energy balance, by the small molecule GSK621 induces calreticulin (CALR) membrane exposure in murine and human AML cells. When CALR is exposed on the cell surface, it serves as a damage-associated molecular pattern that stimulates immune responses. We found that GSK621-treated murine leukemia cells promote the activation and maturation of bone marrow-derived dendritic cells. Moreover, vaccination with GSK621-treated leukemia cells had a protective effect in syngeneic immunocompetent recipients bearing transplanted AMLs. This effect was lost in recipients depleted of CD4/CD8 T cells. Together, these results demonstrate that AMPK activation by GSK621 elicits traits of immunogenic cell death and promotes a robust immune response against leukemia. Pharmacologic AMPK activation thus represents a new potential target for improving the activity of immunotherapy in AML.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: J.M. was funded by the French National Institute for Health and Medical Research (INSERM, Plan Cancer InCa, formation à la recherche translationnelle en cancérologie), the Ligue Contre le Cancer, the Monahan Foundation (partner of the Fullbright Foundation), L’Oréal UNESCO for Women in Science French Young Talents program, and the Philippe Foundation. M.G. was funded by the Cancéropôle Grand Sud-Ouest. M.G., L.P., and J.-E.S. report funding from the Laboratoire d'Excellence Toulouse Cancer (TOUCAN and TOUCAN2.0; contract ANR11-LABEX), the French Institut National du Cancer (PLBIO 2020-010), the Fondation ARC, and the Ligue Contre le Cancer. O.K. reports grants from the French Institut National du Cancer and the DIM Elicit initiative of the Ile de France and is a cofounder of Samsara Therapeutics. A.A.L. was funded by the National Institutes of Health/National Cancer Institute (CA225191), the Ludwig Center at Harvard, Alex’s Lemonade Stand Foundation, and is a scholar of the Leukemia & Lymphoma Society. G.K. declares having held research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Osasuna Therapeutics, Samsara Therapeutics, Sanofi, Sotio, Tollys, Vascage, and Vasculox/Tioma; has received consulting/advisory honoraria from Reithera; serves on the board of directors of the Bristol Myers Squibb Foundation France; is a scientific cofounder of everImmune, Osasuna Therapeutics, Samsara Therapeutics, and Therafast Bio; is the inventor of patents covering therapeutic targeting of aging, cancer, cystic fibrosis, and metabolic disorders; has received research funding from AbbVie and Stemline Therapeutics; and received consulting fees from Adaptimmune, Cimeio, IDRx, N-of-one, Qiagen, and Stemline Therapeutics. The remaining authors declare no competing financial interests.
Figures

Similar articles
-
Natural Killer Immunotherapy for Minimal Residual Disease Eradication Following Allogeneic Hematopoietic Stem Cell Transplantation in Acute Myeloid Leukemia.Int J Mol Sci. 2019 Apr 26;20(9):2057. doi: 10.3390/ijms20092057. Int J Mol Sci. 2019. PMID: 31027331 Free PMC article. Review.
-
CD38-directed CAR-T cell therapy: a novel immunotherapy strategy for relapsed acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation.J Hematol Oncol. 2021 May 25;14(1):82. doi: 10.1186/s13045-021-01092-4. J Hematol Oncol. 2021. PMID: 34034795 Free PMC article.
-
Immune-Based Therapeutic Interventions for Acute Myeloid Leukemia.Cancer Treat Res. 2022;183:225-254. doi: 10.1007/978-3-030-96376-7_8. Cancer Treat Res. 2022. PMID: 35551662
-
Novel Immune Cell-Based Therapies to Eradicate High-Risk Acute Myeloid Leukemia.Front Immunol. 2021 Aug 3;12:695051. doi: 10.3389/fimmu.2021.695051. eCollection 2021. Front Immunol. 2021. PMID: 34413848 Free PMC article. Review.
-
TRAIL expression by activated human CD4(+)V alpha 24NKT cells induces in vitro and in vivo apoptosis of human acute myeloid leukemia cells.Blood. 2001 Apr 1;97(7):2067-74. doi: 10.1182/blood.v97.7.2067. Blood. 2001. PMID: 11264173
Cited by
-
A novel immunogenic cell death-related classification indicates the immune landscape and predicts clinical outcome and treatment response in acute myeloid leukemia.Cancer Cell Int. 2024 Apr 16;24(1):139. doi: 10.1186/s12935-024-03326-0. Cancer Cell Int. 2024. PMID: 38627685 Free PMC article.
References
-
- DiNardo CD, Wei AH. How I treat acute myeloid leukemia in the era of new drugs. Blood. 2020;135(2):85–96. - PubMed
-
- Bokhari SW, Watson L, Nagra S, et al. Role of HCT-comorbidity index, age and disease status at transplantation in predicting survival and non-relapse mortality in patients with myelodysplasia and leukemia undergoing reduced-intensity-conditioning hemopoeitic progenitor cell transplantation. Bone Marrow Transplant. 2012;47(4):528–534. - PubMed
-
- Carré M, Porcher R, Finke J, et al. Role of age and hematopoietic cell transplantation-specific comorbidity index in myelodysplastic patients undergoing an allotransplant: a retrospective study from the Chronic Malignancies Working Party of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2020;26(3):451–457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

