Mathematical Models of HIV-1 Dynamics, Transcription, and Latency
- PMID: 37896896
- PMCID: PMC10612035
- DOI: 10.3390/v15102119
Mathematical Models of HIV-1 Dynamics, Transcription, and Latency
Abstract
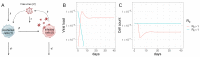
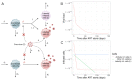
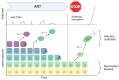
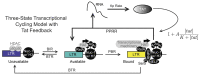
HIV-1 latency is a major barrier to curing infections with antiretroviral therapy and, consequently, to eliminating the disease globally. The establishment, maintenance, and potential clearance of latent infection are complex dynamic processes and can be best described with the help of mathematical models followed by experimental validation. Here, we review the use of viral dynamics models for HIV-1, with a focus on applications to the latent reservoir. Such models have been used to explain the multi-phasic decay of viral load during antiretroviral therapy, the early seeding of the latent reservoir during acute infection and the limited inflow during treatment, the dynamics of viral blips, and the phenomenon of post-treatment control. Finally, we discuss that mathematical models have been used to predict the efficacy of potential HIV-1 cure strategies, such as latency-reversing agents, early treatment initiation, or gene therapies, and to provide guidance for designing trials of these novel interventions.
Keywords: HIV-1; latency; mathematical modeling; reactivation; stochastic fluctuations; transcription.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Mathematical Models of HIV Latency.Curr Top Microbiol Immunol. 2018;417:131-156. doi: 10.1007/82_2017_77. Curr Top Microbiol Immunol. 2018. PMID: 29164341 Free PMC article. Review.
-
Stochastic population switch may explain the latent reservoir stability and intermittent viral blips in HIV patients on suppressive therapy.J Theor Biol. 2014 Nov 7;360:137-148. doi: 10.1016/j.jtbi.2014.06.042. Epub 2014 Jul 10. J Theor Biol. 2014. PMID: 25016044
-
Modeling latently infected cell activation: viral and latent reservoir persistence, and viral blips in HIV-infected patients on potent therapy.PLoS Comput Biol. 2009 Oct;5(10):e1000533. doi: 10.1371/journal.pcbi.1000533. Epub 2009 Oct 16. PLoS Comput Biol. 2009. PMID: 19834532 Free PMC article.
-
Posttranscriptional Regulation of HIV-1 Gene Expression during Replication and Reactivation from Latency by Nuclear Matrix Protein MATR3.mBio. 2018 Nov 13;9(6):e02158-18. doi: 10.1128/mBio.02158-18. mBio. 2018. PMID: 30425153 Free PMC article.
-
Modeling HIV persistence, the latent reservoir, and viral blips.J Theor Biol. 2009 Sep 21;260(2):308-31. doi: 10.1016/j.jtbi.2009.06.011. Epub 2009 Jun 17. J Theor Biol. 2009. PMID: 19539630 Free PMC article. Review.
Cited by
-
Distinguishable topology of the task-evoked functional genome networks in HIV-1 reservoirs.iScience. 2024 Oct 21;27(11):111222. doi: 10.1016/j.isci.2024.111222. eCollection 2024 Nov 15. iScience. 2024. PMID: 39559761 Free PMC article.
-
QuickFit: A High-Throughput RT-qPCR-Based Assay to Quantify Viral Growth and Fitness In Vitro.Viruses. 2024 Aug 19;16(8):1320. doi: 10.3390/v16081320. Viruses. 2024. PMID: 39205294 Free PMC article.
References
-
- Finzi D., Hermankova M., Pierson T., Carruth L.M., Buck C., Chaisson R.E., Quinn T.C., Chadwick K., Margolick J., Brookmeyer R., et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. - DOI - PubMed
-
- Finzi D., Blankson J., Siliciano J.D., Margolick J.B., Chadwick K., Pierson T., Smith K., Lisziewicz J., Lori F., Flexner C., et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 1999;5:512–517. doi: 10.1038/8394. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

