The Crosstalk between Mesenchymal Stromal/Stem Cells and Hepatocytes in Homeostasis and under Stress
- PMID: 37894893
- PMCID: PMC10607347
- DOI: 10.3390/ijms242015212
The Crosstalk between Mesenchymal Stromal/Stem Cells and Hepatocytes in Homeostasis and under Stress
Abstract
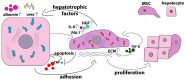
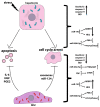
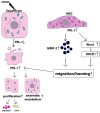
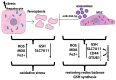
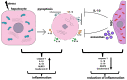
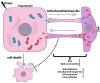
Liver diseases, characterized by high morbidity and mortality, represent a substantial medical problem globally. The current therapeutic approaches are mainly aimed at reducing symptoms and slowing down the progression of the diseases. Organ transplantation remains the only effective treatment method in cases of severe liver pathology. In this regard, the development of new effective approaches aimed at stimulating liver regeneration, both by activation of the organ's own resources or by different therapeutic agents that trigger regeneration, does not cease to be relevant. To date, many systematic reviews and meta-analyses have been published confirming the effectiveness of mesenchymal stromal cell (MSC) transplantation in the treatment of liver diseases of various severities and etiologies. However, despite the successful use of MSCs in clinical practice and the promising therapeutic results in animal models of liver diseases, the mechanisms of their protective and regenerative action remain poorly understood. Specifically, data about the molecular agents produced by these cells and mediating their therapeutic action are fragmentary and often contradictory. Since MSCs or MSC-like cells are found in all tissues and organs, it is likely that many key intercellular interactions within the tissue niches are dependent on MSCs. In this context, it is essential to understand the mechanisms underlying communication between MSCs and differentiated parenchymal cells of each particular tissue. This is important both from the perspective of basic science and for the development of therapeutic approaches involving the modulation of the activity of resident MSCs. With regard to the liver, the research is concentrated on the intercommunication between MSCs and hepatocytes under normal conditions and during the development of the pathological process. The goals of this review were to identify the key factors mediating the crosstalk between MSCs and hepatocytes and determine the possible mechanisms of interaction of the two cell types under normal and stressful conditions. The analysis of the hepatocyte-MSC interaction showed that MSCs carry out chaperone-like functions, including the synthesis of the supportive extracellular matrix proteins; prevention of apoptosis, pyroptosis, and ferroptosis; support of regeneration; elimination of lipotoxicity and ER stress; promotion of antioxidant effects; and donation of mitochondria. The underlying mechanisms suggest very close interdependence, including even direct cytoplasm and organelle exchange.
Keywords: apoptosis; cell-to-cell communication; ferroptosis; hepatocytes; lipotoxicity; liver diseases; mesenchymal stem cells; mitochondrial transfer; pyroptosis; regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Mesenchymal stromal cells protect hepatocytes from lipotoxicity through alleviation of endoplasmic reticulum stress by restoring SERCA activity.J Cell Mol Med. 2021 Mar;25(6):2976-2993. doi: 10.1111/jcmm.16338. Epub 2021 Feb 16. J Cell Mol Med. 2021. PMID: 33591626 Free PMC article.
-
Administration of multipotent mesenchymal stromal cells restores liver regeneration and improves liver function in obese mice with hepatic steatosis after partial hepatectomy.Stem Cell Res Ther. 2017 Jan 28;8(1):20. doi: 10.1186/s13287-016-0469-y. Stem Cell Res Ther. 2017. PMID: 28129776 Free PMC article.
-
Exosomes from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells (hiPSC-MSCs) Protect Liver against Hepatic Ischemia/ Reperfusion Injury via Activating Sphingosine Kinase and Sphingosine-1-Phosphate Signaling Pathway.Cell Physiol Biochem. 2017;43(2):611-625. doi: 10.1159/000480533. Epub 2017 Sep 21. Cell Physiol Biochem. 2017. PMID: 28934733
-
The multiple functional roles of mesenchymal stem cells in participating in treating liver diseases.J Cell Mol Med. 2015 Mar;19(3):511-20. doi: 10.1111/jcmm.12482. Epub 2014 Dec 23. J Cell Mol Med. 2015. PMID: 25534251 Free PMC article. Review.
-
Hepatocyte differentiation of mesenchymal stem cells.Hepatobiliary Pancreat Dis Int. 2012 Aug 15;11(4):360-71. doi: 10.1016/s1499-3872(12)60193-3. Hepatobiliary Pancreat Dis Int. 2012. PMID: 22893462 Review.
Cited by
-
Prognostic role of the stromal cell derived factor-1 in patients with hepatitis B virus-related acute-on-chronic liver failure.World J Clin Cases. 2024 Jul 6;12(19):3845-3853. doi: 10.12998/wjcc.v12.i19.3845. World J Clin Cases. 2024. PMID: 38994298 Free PMC article.
-
Mechanism of mesenchymal stem cells in liver regeneration: Insights and future directions.World J Stem Cells. 2024 Sep 26;16(9):842-845. doi: 10.4252/wjsc.v16.i9.842. World J Stem Cells. 2024. PMID: 39351263 Free PMC article.
-
ROS-responsive MSC-derived Exosome Mimetics Carrying MHY1485 Alleviate Renal Ischemia Reperfusion Injury through Multiple Mechanisms.ACS Omega. 2024 May 28;9(23):24853-24863. doi: 10.1021/acsomega.4c01624. eCollection 2024 Jun 11. ACS Omega. 2024. PMID: 38882096 Free PMC article.
-
Hepatic Macrophages as Targets for the MSC-Based Cell Therapy in Non-Alcoholic Steatohepatitis.Biomedicines. 2023 Nov 14;11(11):3056. doi: 10.3390/biomedicines11113056. Biomedicines. 2023. PMID: 38002056 Free PMC article. Review.
References
-
- Pimpin L., Cortez-Pinto H., Negro F., Corbould E., Lazarus J.V., Webber L., Sheron N., EASL HEPAHEALTH Steering Committee Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J. Hepatol. 2018;69:718–735. doi: 10.1016/j.jhep.2018.05.011. - DOI - PubMed
-
- Karlsen T.H., Sheron N., Zelber-Sagi S., Carrieri P., Dusheiko G., Bugianesi E., Pryke R., Hutchinson S.J., Sangro B., Martin N.K., et al. The EASL-Lancet Liver Commission: Protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet. 2022;399:61–116. doi: 10.1016/S0140-6736(21)01701-3. - DOI - PubMed
-
- GBD 2017 Cirrhosis Collaborators The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020;5:245–266. doi: 10.1016/S2468-1253(19)30349-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

