Interaction of Calmodulin with TRPM: An Initiator of Channel Modulation
- PMID: 37894842
- PMCID: PMC10607381
- DOI: 10.3390/ijms242015162
Interaction of Calmodulin with TRPM: An Initiator of Channel Modulation
Abstract
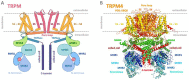
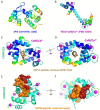
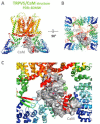
Transient receptor potential melastatin (TRPM) channels, a subfamily of the TRP superfamily, constitute a diverse group of ion channels involved in mediating crucial cellular processes like calcium homeostasis. These channels exhibit complex regulation, and one of the key regulatory mechanisms involves their interaction with calmodulin (CaM), a cytosol ubiquitous calcium-binding protein. The association between TRPM channels and CaM relies on the presence of specific CaM-binding domains in the channel structure. Upon CaM binding, the channel undergoes direct and/or allosteric structural changes and triggers down- or up-stream signaling pathways. According to current knowledge, ion channel members TRPM2, TRPM3, TRPM4, and TRPM6 are directly modulated by CaM, resulting in their activation or inhibition. This review specifically focuses on the interplay between TRPM channels and CaM and summarizes the current known effects of CaM interactions and modulations on TRPM channels in cellular physiology.
Keywords: TRPM channels; calcium homeostasis; calmodulin; calmodulin binding site; regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Ca2+-dependent regulation and binding of calmodulin to multiple sites of Transient Receptor Potential Melastatin 3 (TRPM3) ion channels.Cell Calcium. 2018 Jul;73:40-52. doi: 10.1016/j.ceca.2018.03.005. Epub 2018 Mar 31. Cell Calcium. 2018. PMID: 29880196
-
Calmodulin and S100A1 protein interact with N terminus of TRPM3 channel.J Biol Chem. 2012 May 11;287(20):16645-55. doi: 10.1074/jbc.M112.350686. Epub 2012 Mar 27. J Biol Chem. 2012. PMID: 22451665 Free PMC article.
-
Function and pharmacology of TRPM cation channels.Naunyn Schmiedebergs Arch Pharmacol. 2005 Apr;371(4):307-14. doi: 10.1007/s00210-005-1034-x. Naunyn Schmiedebergs Arch Pharmacol. 2005. PMID: 15843919 Review.
-
Shared CaM- and S100A1-binding epitopes in the distal TRPM4 N terminus.FEBS J. 2018 Feb;285(3):599-613. doi: 10.1111/febs.14362. Epub 2017 Dec 29. FEBS J. 2018. PMID: 29240297
-
Multiple roles of calmodulin and other Ca(2+)-binding proteins in the functional regulation of TRP channels.Pflugers Arch. 2005 Oct;451(1):105-15. doi: 10.1007/s00424-005-1427-1. Epub 2005 May 28. Pflugers Arch. 2005. PMID: 15924238 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

